| Identification | More | [Name]
Prednisone | [CAS]
53-03-2 | [Synonyms]
1,4-PREGNADIEN-17,21-DIOL-3,11,20-TRIONE
1,4-PREGNADIEN-17ALPHA,21-DIOL-3,11,20-TRIONE
1,4-PREGNADIENE-17ALPHA,21-DIOL-3,11,20-TRIONE
17ALPHA,21-DIHYDROXY-1,4-PREGNADIENE-3,11,20-TRIONE
17ALPHA,21-DIHYDROXYPREGNA-1,4-DIENE-3,11,20-TRIONE
1-CORTISONE
1-DEHYDROCORTISONE
(8S,9S,10R,13S,14S,17R)-17-HYDROXY-17-(2-HYDROXY-ACETYL)-10,13-DIMETHYL-7,8,9,10,12,13,14,15,16,17-DECAHYDRO-6H-CYCLOPENTA[A]PHENANTHRENE-3,11-DIONE
DEHYDROCORTISONE
DELTA1-CORTISONE
PREDNISON
PREDNISONE
PREDNISONUM
1,2-Dehydrocortisone
1,4-pregnadiene-16-alpha,21-diol-3,11,20-trione
11,20-trione,17,21-dihydroxy-pregna-4-diene-3
11,20-trione,17,21-hydroxy-pregna-4-diene-3
Ancortone
Bicortone
Colisone | [EINECS(EC#)]
200-160-3 | [Molecular Formula]
C21H26O5 | [MDL Number]
MFCD00003608 | [Molecular Weight]
358.43 | [MOL File]
53-03-2.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline powder | [Melting point ]
236-238 °C(lit.)
| [alpha ]
169 º (c=0.5, dioxane) | [Boiling point ]
410.86°C (rough estimate) | [density ]
1.1121 (rough estimate) | [refractive index ]
170 ° (C=0.5, Dioxane) | [Fp ]
>200℃ | [storage temp. ]
2-8°C | [solubility ]
Practically insoluble in water, slightly soluble in ethanol (96 per cent) and in methylene chloride. It shows polymorphism (5.9). | [form ]
Solid | [pka]
12.36±0.60(Predicted) | [color ]
White to Off-White | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [Water Solubility ]
115mg/L(25 ºC) | [Merck ]
7722 | [BRN ]
2065301 | [BCS Class]
1? | [CAS DataBase Reference]
53-03-2(CAS DataBase Reference) | [IARC]
3 (Vol. 26, Sup 7) 1987 | [NIST Chemistry Reference]
Prednisone(53-03-2) | [EPA Substance Registry System]
53-03-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R63:Possible risk of harm to the unborn child.
R34:Causes burns.
R11:Highly Flammable. | [Safety Statements ]
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S16:Keep away from sources of ignition-No smoking . | [Safety Profile]
Poison by
intraperitoneal and subcutaneous routes.
Moderately toxic by intramuscular route.
Human systemic effects: sensory change
involving peripheral nerves, dermatitis.
Experimental reproductive effects.
Questionable carcinogen with experimental
tumorigenic data. Mutation data reported.
Has been implicated in aplastic anemia. | [Hazardous Substances Data]
53-03-2(Hazardous Substances Data) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Selenium dioxide-->Hydrocortisone-->Cortisone acetate | [Preparation Products]
Deprodone propionate-->11-alpha,17-alpha,21-trihydroxypregn-4-ene-3,20-dione-->Pregna-1,4-diene-3,11,20-trione, 17-hydroxy--->17,20:20,21-Bis[Methylenebis(oxy)]-pregna-1,4-diene-3,11-dione-->CORTISONE |
| Hazard Information | Back Directory | [General Description]
Odorless white crystalline powder. | [Air & Water Reactions]
Very slightly water soluble . | [Hazard]
Questionable carcinogen.
| [Chemical Properties]
White or almost white, crystalline powder. | [Uses]
Adrenocortical steroid. Glucocorticoid, antiinflammatory. | [Uses]
Downregulates TNF-α production and NF-κB expression | [Uses]
Prednisone is used for the same indications as cortisone for inflammatory processes,
allergies, and adrenal gland insufficiency; however, it is somewhat more active than cortisone
and has less of an effect on mineral volume. | [Definition]
ChEBI: A synthetic glucocorticoid drug that is particularly effective as an immunosuppressant, and affects virtually all of the immune system. Prednisone is a prodrug that is converted by the liver into prednisolone (a beta-hydroxy group instead
f the oxo group at position 11), which is the active drug and also a steroid. | [Indications]
Prednisone 2.5 to 7.5 mg, administered at night or dexamethasone 0.25
to 0.75 mg are useful in female patients, with severe acne unresponsive
to conventional therapy, who suffer from adrenal gland overproduction
of androgens such as congenital adrenal hyperplasia. | [Brand name]
Cortan (Halsey); Delta-Dome (Bayer); Deltasone (Pharmacia & Upjohn); Liquid Pred (Muro); Meticorten (Schering); Orasone (Solvay Pharmaceuticals); Paracort (Parke-Davis). | [Synthesis]
Prednisone is 17|á,21-dihydroxypregna-1,4-dien-3,11-20-trione (27.1.31).
Prednisone differs from cortisone in the presence of an additional double bond between C1
and C2. There are various ways of synthesizing it. In one of these, as is in the case when
synthesizing cortisone, it is synthesized from dihydrocortisone acetate. However, in the
given example, this compound undergoes dibromination by molecular bromine, giving
2,4-dibromo-derivative of dihydrocortisone 27.1.30. Dehydrobromination with
3,5-lutidine, followed by subsequent hydrolysis of the acetyl group using potassium bicarbonate
gives the desired prednisone (27.1.31). Prednisone is also synthesized by
microbiological dehydrogenation of cortisone.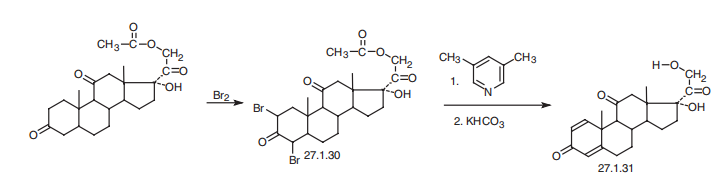
| [Purification Methods]
Crystallise prednisone from acetone/hexane, then recrystallise it from Me2CO. The monoacetate crystallises from Me2CO/hexane with m 227-233o(dec), [�] D +186o (c 1, dioxane), and the diacetate crystallises from 25Me2CO/hexane with m 219-221o(dec), [�] D +125o (c 1, CHCl3). [Hertzog et al. Tetrahedron 18 581 1962, Beilstein 8 IV 3531.] |
|
|