| Identification | More | [Name]
5-Bromo-2-chlorobenzoic acid | [CAS]
21739-92-4 | [Synonyms]
2-CHLORO-5-BROMOBENZOIC ACID
5-BROMO-2-CHLOROBENZOIC ACID
Benzoic acid, 5-bromo-2-chloro-
5-Bromo-2-chlorobenzoic acid, 98+%
5-Bromo-2-Chlorobenzoic Acid (BCBA) | [EINECS(EC#)]
244-558-5 | [Molecular Formula]
C7H4BrClO2 | [MDL Number]
MFCD00002415 | [Molecular Weight]
235.46 | [MOL File]
21739-92-4.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powder | [Melting point ]
154-156 °C (lit.) | [Boiling point ]
324.5±27.0 °C(Predicted) | [density ]
1.7312 (rough estimate) | [refractive index ]
1.5590 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
2.63g/l | [form ]
Solid | [pka]
2.49±0.25(Predicted) | [color ]
Off white colored powder | [Water Solubility ]
Soluble in water 2.63 g/L @20°C. | [BRN ]
2691432 | [InChI]
InChI=1S/C7H4BrClO2/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3H,(H,10,11) | [InChIKey]
FGERXQWKKIVFQG-UHFFFAOYSA-N | [SMILES]
C(O)(=O)C1=CC(Br)=CC=C1Cl | [CAS DataBase Reference]
21739-92-4(CAS DataBase Reference) | [NIST Chemistry Reference]
5-Bromo-2-chlorobenzoic acid(21739-92-4) | [EPA Substance Registry System]
Benzoic acid, 5-bromo-2-chloro- (21739-92-4) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT | [HS Code ]
29163900 |
| Raw materials And Preparation Products | Back Directory | [Preparation Products]
5'-BROMO-2'-CHLOROACETOPHENONE-->5-BROMO-2-CHLORO-BENZOYL CHLORIDE-->Bexagliflozin-->ETHYL 5-BROMO-2-CHLOROBENZOATE-->4-(5-broMo-2-chlorobenzyl)phenol-->2-(5-Bromo-2-chloro-phenyl)-1H-benzoimidazole-->(5-bromo-2-chlorophenyl)(4-fluorophenyl)methanone-->5-Bromo-2-chloro-N-cyclopropylbenzamide-->[1,1'-Biphenyl]-3-carboxylic acid, 4-chloro-3'-fluoro--->2,5-Bis(2,2,2-trifluoroethoxy)benzoic acid-->(2S,3R,4R,5S,6R)-2-(4-chloro-3-((4-ethoxyphenyl)(methoxy) methyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol-->(5-bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone |
| Hazard Information | Back Directory | [Description]
5-Bromo-2-chlorobenzoic acid (BCBA) is an organic reagent that can be used in electroinduced dehalogenation reactions. Ag/Cu electrodes exhibit high electrocatalytic activity in the dehalogenation reaction of BCBA. The process is characterised by a sequence of halide excretion and hydrogen addition. Since the bond dissociation energy value of C-Br bond is smaller than that of C-Cl bond, Br-w is more easily released from the benzene ring. As C-Br breaks, BCBA gains electrons and hydrogen to form the intermediate 2-chlorobenzoate. C-Cl then breaks in the same way to give the final product benzoate.
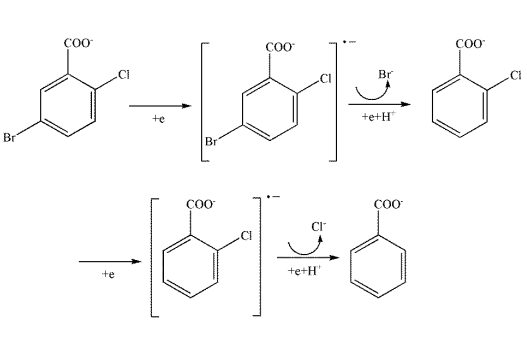 | [Chemical Properties]
white to light yellow crystal powder | [Uses]
5-Bromo-2-chlorobenzoic acid was used to evaluate the homogeneity of organic powder. It can be used to produce 2-chloro-5-deuteriobenzoic acid. | [Synthesis]
4.7g (0.03 mol) of 2-chlorobenzoic acid, 40mL of concentrated sulfuric acid and 0.936g (0.012 mol) of sodium sulfide are sequentially added into a 250mL four-mouth bottle, stirred for 20 minutes at 30 ℃ until the solution is clear, then 5.334g N-bromosuccinimide (0.03 mol) is added, the reaction is continued for 10 minutes at 30 ℃, and then the solution is slowly poured into 80mL ice water bath for crystallization to obtain a crude product of the 5-bromo-2-chlorobenzoic acid. Add the filter cake into a 250mL four-mouth bottle, add 24mL methanol and 36mL water, heat to 60 ℃, naturally cool, and stir for crystallization. Filtration, washing with 20mL of 40% volume fraction aqueous methanol, and drying at 55 ℃ for 6 hours gave 6.001g of a white solid, yield: 85.0%. | [References]
[1] Fen Wang . “The Electroreductive Dehalogenation Reaction of 5-Bromo-2-chlorobenzoic Acid on Dendritic Ag/Cu.” International Journal of Electrochemical Science 10 6 (2015): Pages 5101-5111.
|
|
|