| Identification | More | [Name]
2-(7-Aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate | [CAS]
148893-10-1 | [Synonyms]
1-[BIS(DIMETHYLAMINO)METHYLENE]-1 H-1,2,3-TRIAZOLO(4,5-B) PYRIDINIUM 3-OXIDE HEXAFLUOROPHOSPHATE
2-(1H-7-AZABENZOTRIAZOL-1-YL)-1,1,3,3-TETRAMETHYL URONIUM HEXAFLUOROPHOSPHATE METHANAMINIUM
2-(7-AZA-1H-BENZOTRIAZOLE-1-YL)-1,1,3,3-TETRAMETHYLURONIUM HEXAFLUOROPHOSPHATE
HATU
N-[(DIMETHYLAMINO)-1H-1,2,3-TRIAZOLO[4,5-B]PYRIDINO-1-YLMETHYLENE]-N-METHYLMETHANAMINIUM HEXAFLUOROPHOSPHATE N-OXIDE
N,N,N',N'-TETRAMETHYL-O-(7-AZABENZOTRIAZOL-1-YL)URONIUM HEXAFLUOROPHOSPATE
N,N,N',N'-TETRAMETHYL-O-(7-AZABENZOTRIAZOL-1-YL)URONIUM HEXAFLUOROPHOSPHATE
O-(7-AZABENZOTRIAZOL-1-YL)-1,1,3,3-TETRAMETHYLURONIUM HEXAFLUOROPHOSPHATE
O-(7-AZABENZOTRIAZOL-1-YL)-N,N,N',N'-TETRAMETHYLURONIUM HEXAFLUOROPHOSPHATE
O-(7-AZABENZOTRIAZOL-1-YL)URONIUM HEXAFLUORO-PHOSPHATE
O-(7-AZABENZOTRIAZOLE-1-YL)-N, N,N',N'-TETRAMETHYLURONIUM HEXAFLUOROPHOSPHATE
o-(7-azabenzotriazol-1-yl)-n,n,n’,n’-te-tramethyluroniumpf6
O-(7-AZABENZOTRIAZOL-1-YL)-N,N,N',N'-TE&
HATU 2-(7-AZOBENZOTRIAZOLYL-1-OXY)-N,N,N',N'-TETRAMETHYLURONIUM HEXAFLUOROPHOSPHATE 99%
2-(7-AZA-1H-BENZOTRIAZOLE-1-YL)-1,1,3,3-TETRAMETHYLURONIUM HEXAFLUOROPHOSPHATE (HATU)
O-(7-Azabenzotriazol-1-yl)-N,N,N',N'-tetramethylur
O-(7-AZABENZOTRIAZOL-1-YL)-N,N,N',N'-TETRAMETHYLURONIUM HEXAFLUOROPHOSPHATE (HATU)
O-(7-AZABENZOTRIAZOL-1-YL)-1,1,3,3-TETRAMETHYLURONIUM HEXAFLUOROPHOSPHATE (HATU)
HATU O-(7-AZABENZOTRIAZOL-1-YL)-N,N,N'',N''-TETRAMETHYLURONIUM HEXAFLUOROPHOSPHATE
O-(7-AZABENZOTRIAZOL-1-YL)-N,N,N',N'-TETRAMETHYLUROINIUM HEXAFLUOROPHOSPHATE | [EINECS(EC#)]
604-662-7 | [Molecular Formula]
C10H16F6N6OP | [MDL Number]
MFCD00274639 | [Molecular Weight]
381.24 | [MOL File]
148893-10-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White crystalline to off-white powder | [Melting point ]
183-188 °C (dec.)
| [RTECS ]
XZ5633000 | [storage temp. ]
2-8°C
| [solubility ]
>16mg/mL in DMSO | [form ]
powder to crystaline | [color ]
White to Almost white | [Water Solubility ]
Soluble in acetonitrile. Insoluble in water. | [InChI]
InChI=1S/C10H15N6O.F6P/c1-13(2)10(14(3)4)15-8-6-5-7-11-9(8)16(17)12-15;1-7(2,3,4,5)6/h5-7H,1-4H3;/q+1;-1 | [InChIKey]
KZAWCZZRROLLDL-UHFFFAOYSA-N | [SMILES]
[P+5]([F-])([F-])([F-])([F-])([F-])[F-].C(=[N+]1/N=N(=O)C2=NC=CC=C/12)(\N(C)C)/N(C)C | [CAS DataBase Reference]
148893-10-1(CAS DataBase Reference) | [EPA Substance Registry System]
148893-10-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection .
S36/37:Wear suitable protective clothing and gloves .
S36:Wear suitable protective clothing . | [RIDADR ]
1325 | [WGK Germany ]
3
| [F ]
10-21 | [HazardClass ]
4.1 | [PackingGroup ]
Ⅱ | [HS Code ]
29339900 |
| Hazard Information | Back Directory | [Description]
HATU, first prepared by Louis A. Carpino in 1993, is widely used in carboxylic acid amidation reactions. It acts as a facilitator of amide bond generation by activating the carboxyl group. | [Chemical Properties]
White crystalline to off-white powder | [Uses]
A peptide coupling reagent | [Reactions]
HATU is a very promising coupling agent for chemical protein synthesis.
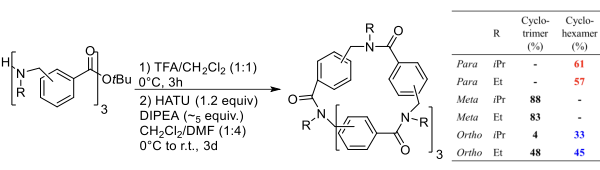
This strategy was exploited to prepare macrocycles from the trimeric linear arylopeptoids (ortho-, meta-, and para-) containing isopropyl or ethyl side chains, synthesized as described by Hjelmgaard et al. The cyclization procedure reported for α,β-cyclopeptoids was applied. The linear arylopeptoids were cyclized in the presence of HATU and DIPEA in CH?Cl?/DMF (4:1) after the deprotection of the tert-butyl group in TFA/CH?Cl?.
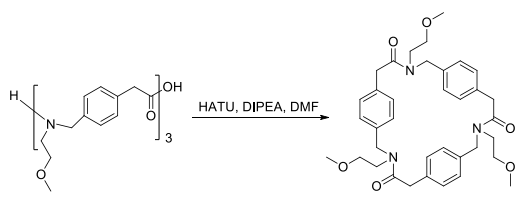
After the synthesis of the Fmoc-protected monomers, the oligomers were synthesized on the 2-chlorotrityl resin with excellent yield of coupling (>98%). The trimers and tetramers of the different isomers were synthesized in good overall yield (60-84%). Then, the crude oligomers were cyclized in DMF in the presence of HATU and DIPEA in high dilution (3 × 10?3 M) to furnish the cyclized trimers and tetramers in good yields ranging between 32% and 72%. | [Synthesis]
The synthesis of HATU is as follows:The resulting residue treated with 2-Methoxycarbonylamino-3-methyl-butyric acid (60 mg, 0.343 mmol) and HATU (130 mg, 0.343 mmol), suspended in DMF (3 mL) and cooled to 0° C. DIPEA (0.272 mL, 1.56 mmol) was added dropwise. After stirring for 4 h, NaOH (5M in H2O, 0.300 mL, 1.5 mmol) was added. This mixture was stirred for 3 h then diluted with EtOAc and washed with 1 M LiOH (2*) then brine. The organic phase was dried over MgSO4, filtered and concentrated. The crude residue was then purified by HPLC to afford the title compound (53 mg, 44%).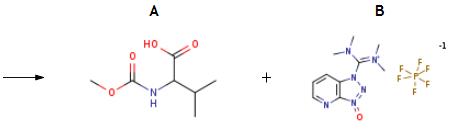
| [References]
[1] CHAWLA P A, SHOME A, JHA K T. Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium (HATU): A Unique Cross-Coupling Reagent[J]. SynOpen, 2023, 66 2: 0. DOI:10.1055/s-0042-1751499.
[2] LOUIS A. CARPINO. Comparison of the Effects of 5- and 6-HOAt on Model Peptide Coupling Reactions Relative to the Cases for the 4- and 7-Isomers?,?[J]. Organic Letters, 2000, 2 15: 2253-2256. DOI:10.1021/ol006013z. |
|
|