| Identification | More | [Name]
1,2,4-Triazole | [CAS]
288-88-0 | [Synonyms]
1,2,4-1H-TRIAZOLE
1H-1,2,4-TRIAZOLE
1-HYDRO-1,2,4-TRIAZOLE
AKOS BBS-00004410
PYRRODIAZOLE
TA-4
TRIAZOLE
TRIAZOLE(1,2,4-)
1,2,4-Triazol
1H-1,2,4-Triazol
4H-1,2,4-triazole
CGA-71019
s-Triazole
1,2,4-TRIAZOLE, 1GM, NEAT
1,2,4-TriazoleForSynthesis
1,2,4-Triazole,99%
1,2,4-1H-Triazole, 99.50%
1,2,4-1H-Triazole, flakes, 99+%
pyrrodiazol
1H-1,2,4-TRIAZLOENA-1,2,4-TRIAZLOE K-1,2,4-TRIAZLO | [EINECS(EC#)]
206-022-9 | [Molecular Formula]
C2H3N3 | [MDL Number]
MFCD00005228 | [Molecular Weight]
69.07 | [MOL File]
288-88-0.mol |
| Chemical Properties | Back Directory | [Appearance]
white flakes | [Melting point ]
119-121 °C (lit.) | [Boiling point ]
260 °C (lit.) | [bulk density]
550kg/m3 | [density ]
1.15 g/cm3 (130℃) | [vapor pressure ]
0.215Pa at 20℃ | [refractive index ]
1.4854 (estimate) | [Fp ]
140 °C
| [storage temp. ]
Store at 0-5°C | [solubility ]
547g/l | [form ]
Crystalline Powder and Flakes | [pka]
2.27(at 20℃) | [color ]
White | [PH]
8 (10g/l, H2O, 20℃) | [Water Solubility ]
1250 g/L (20 ºC) | [Detection Methods]
T,NMR | [Merck ]
14,9605 | [BRN ]
104767 | [InChIKey]
NSPMIYGKQJPBQR-UHFFFAOYSA-N | [LogP]
-0.76 at 25℃ | [CAS DataBase Reference]
288-88-0(CAS DataBase Reference) | [NIST Chemistry Reference]
1H-1,2,4-Triazole(288-88-0) | [EPA Substance Registry System]
288-88-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi,C | [Risk Statements ]
R22:Harmful if swallowed.
R36:Irritating to the eyes.
R63:Possible risk of harm to the unborn child.
R34:Causes burns. | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S27:Take off immediately all contaminated clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
2
| [RTECS ]
XZ3806000
| [Hazard Note ]
Irritant | [TSCA ]
Yes | [HS Code ]
29339990 | [Hazardous Substances Data]
288-88-0(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 1650 mg/kg LD50 dermal Rabbit 3129 - 4200 mg/kg |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Ammonia-->Hydrazinium hydroxide solution-->Formic acid-->Hydrazine hydrate-->4-Chlorobenzaldehyde-->Ammonium formate-->Methyl formate-->1,2-Diformylhydrazine-->Flutriafol-->1H-1,2,4-Triazole, 1-chloro- | [Preparation Products]
Fluconazole-->Paclobutrazol-->1-[2-(4-CHLOROPHENYL)-1-(1-HYDROXY-1-PHENYLETHYL)ETHYL]-1,2,4-TRIAZOLE-->Anastrozole-->Propiconazole-->1H-1,2,4-Triazole-1-carboximidamide hydrochloride-->Bitertanol-->Triapenthenol-->BASIC RED 46-->1-(4-NITROBENZENESULFONYL)-1H-1,2,4-TRIAZOLE-->3,5-DIBROMO-1H-1,2,4-TRIAZOLE-->3-[(2,4-dihydro-2,4-dimethyl-3H-1,2,4-triazol-3-ylidene)hydrazono]-1-methyl-2-phenyl-3H-indolium chloride-->Basic Red 22-->1-(BETA-D-2-DEOXYRIBOFURANOSYL)-4-(1,2,4-TRIAZOL-1-YL)-5-METHYLPYRIMIDIN-2-ONE-->3-chloro-6-(1H-1,2,4-triazol-1-yl)pyridazine(SALTDATA: FREE)-->Benzaldehyde, 5-chloro-2-(1H-1,2,4-triazol-1-yl)--->1-(2-FLUORO-4-NITROPHENYL)-1H-1,2,4-TRIAZOLE-->4-(1H-1,2,4-TRIAZOL-1-YLMETHYL)BENZALDEHYDE |
| Hazard Information | Back Directory | [Chemical Properties]
white crystalline powder and flakes | [Uses]
1,2,4-Triazole is present in a large variety of fungicides as C14-demethylase-inhibitors. In addition they are used in synthetic chemical reactions such as the design and preparation of ghrelin receptor ligand antagonists, like JMV 2959. | [Definition]
A heterocyclic organic compound with a five-membered ring containing two carbon atoms
and three nitrogen atoms. There are two
isomers. | [Flammability and Explosibility]
Nonflammable | [Synthesis]
Alkyl bromide and sodium azide were used as raw materials to prepare organic azides, and a series of 1,2,3-triazole small molecules were prepared by click chemistry. The structures of the six products were characterized by 1H NMR, 13C NMR and IR. the products were expected targets. Furthermore, the effect of different substituent structure on the reaction was discussed. Especially the introduction of drug molecule ethisterone, which is expected to provide a certain experimental basis for the application of triazole and its derivatives in biomedicine and other fields.
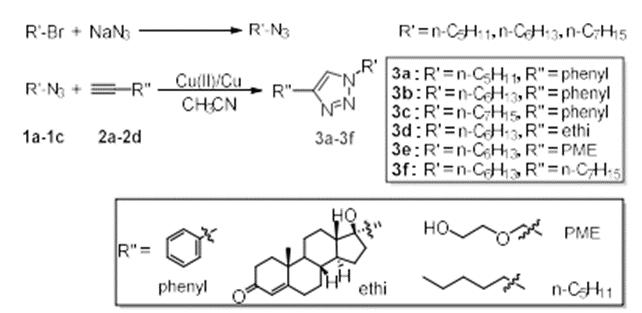 As shown in the figure, alkyl bromide and sodium azide were used as raw materials, N, N-dimethylformamide was used as solvent, and reacted at room temperature for 20 hours under the protection of nitrogen. After the reaction, the solvent was extracted, dried, filtered and spin evaporated to obtain alkyl azide compound 1.Put a certain proportion of alkyl azide 1 and alkynyl compound 2 into a round bottom flask, copper powder and copper acetate as catalysts, acetonitrile as solvent, and react at room temperature for 4 hours under the protection of nitrogen. After that, extract, dry, filter and spin the solvent, and purify by thin chromatography to obtain triazole compound 3. As shown in the figure, alkyl bromide and sodium azide were used as raw materials, N, N-dimethylformamide was used as solvent, and reacted at room temperature for 20 hours under the protection of nitrogen. After the reaction, the solvent was extracted, dried, filtered and spin evaporated to obtain alkyl azide compound 1.Put a certain proportion of alkyl azide 1 and alkynyl compound 2 into a round bottom flask, copper powder and copper acetate as catalysts, acetonitrile as solvent, and react at room temperature for 4 hours under the protection of nitrogen. After that, extract, dry, filter and spin the solvent, and purify by thin chromatography to obtain triazole compound 3.
| [Purification Methods]
Crystallise 1,2,4-triazole from EtOH, H2O, EtOAc (m 120.5-121o), or EtOH/*C6H6. The hydrochloride has m 170o, and the picrate has m 163-164o (from H2O or CHCl3). [Barszcz et al. J Chem Soc, Dalton Trans 2025 1986]. [Beilstein 26 H 13, 26 II 6, 26 III/IV 35.] |
| Spectrum Detail | Back Directory | [Spectrum Detail]
1,2,4-Triazole(288-88-0)MS
1,2,4-Triazole(288-88-0)1HNMR
1,2,4-Triazole(288-88-0)13CNMR
1,2,4-Triazole(288-88-0)IR1
1,2,4-Triazole(288-88-0)IR2
1,2,4-Triazole(288-88-0)Raman
|
|
|