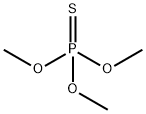
TRIMETHYL THIOPHOSPHATE synthesis
- Product Name:TRIMETHYL THIOPHOSPHATE
- CAS Number:152-18-1
- Molecular formula:C3H9O3PS
- Molecular Weight:156.14
Yield:-
Reaction Conditions:
with 2.9-dimethyl-1,10-phenanthroline;sodium methylate;zinc trifluoromethanesulfonate in methanol at 25;Mechanism;
Steps:
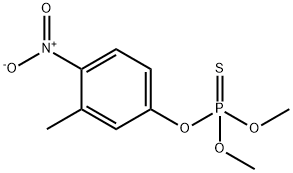
10 Example 10; M2+-Catalyzed Methanolysis of Fenitrothion
The activity of this system may be increased by adding equimolar amounts of bi- or tri-dentate ligands to complex Zn2+(-OCH3) and limit oligomerization of Zn2+(-OCH3)2 in solution. The systems studied herein used methoxide and the ligands phen, diMephen and [12]aneN3. The active forms of the metal ions at neutral sspH are Zn2+(-OCH3) with no added ligand and {Zn2+:L:(-OCH3)} when ligand (L) is present. In the case of phen ligand, decreasing the oligomerization does not prevent the formation Zn2+(-OCH3) dimers since the bulk of the material is now present as {LZn2+(-OCH3)2Zn2+L} which is not catalytically active, but is in equilibrium with an active mononuclear form. The propensity to form the latter inactive dimers can be reduced either by increasing the steric interaction (ligand diMephen) or by changing the coordination number (ligand [12]aneN3) in which cases the overall activity of the catalytic system increases. In the case of ligand diMephen, the dimerization is definitely reduced but the binding to the metal ion is not as strong as in the case of phen or [12]aneN3, which means that there is some free Zn2+ in solution under the concentrations and sspH region where the catalyst is active. [0237] A reaction scheme is given below (Scheme 3) for the methanolysis of fenitrothion where M2+ is a transition metal ion, most preferably Zn2+ or Cu2+. In a preferred embodiment a ligand is present, preferably a bidentate or tridentate ligand, most preferably [12]aneN3 for Cu2+ and diMephen or [12]aneN3 for Zn2+. [C00011] [00011] [0238] As seen in FIGS. 6 and 7, Cu2+:(-OCH3) at 25° C. either alone or in the presence of equimolar [12]aneN3, bpy or phen shows both great catalytic efficacy and specificity toward the PS derivatives. [0239] Apparently matching the hard/soft characteristics of the metal ion and the substrate is important in designing an effective catalytic system for PS substrates. With due consideration for matching the hard/soft characteristics of the substrate and the metal ion, dramatic rate and selectivity can be achieved in the methanolysis of PO vs. PS phosphates.
References:
Brown, R. Stanley;Neverov, Alexei A.;Tsang, Josephine S.W. US2004/230082, 2004, A1 Location in patent:Page 16
15088-78-5
186 suppliers
$6.00/1g
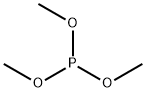
121-45-9
235 suppliers
$14.00/5g

71-36-3
1053 suppliers
$14.00/25mL

152-18-1
66 suppliers
$21.39/1g
122-43-0
91 suppliers
$49.00/100g

121-45-9
235 suppliers
$14.00/5g

152-18-1
66 suppliers
$21.39/1g

121-45-9
235 suppliers
$14.00/5g
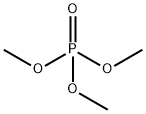
512-56-1
428 suppliers
$15.00/25g

152-18-1
66 suppliers
$21.39/1g
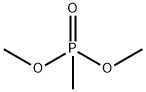
756-79-6
7 suppliers
$15.00/10 g

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)