
Sunitinib synthesis
- Product Name:Sunitinib
- CAS Number:557795-19-4
- Molecular formula:C22H27FN4O2
- Molecular Weight:398.47
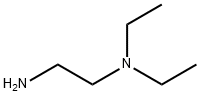
100-36-7
316 suppliers
$14.14/5gm:
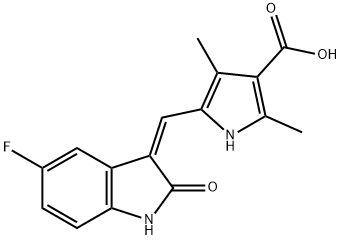
356068-93-4
140 suppliers
$45.00/25mg

557795-19-4
353 suppliers
inquiry
Yield:557795-19-4 70%
Reaction Conditions:
Stage #1: 5-((Z)-(5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxylic acidwith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in tetrahydrofuran at 20; for 0.5 h;
Stage #2: N,N-diethylethylenediamine in tetrahydrofuran at 20;
Steps:
3.3c
To a stirred solution of 5-[(Z)-(5-fluoro-2-oxo4,2-dihydro-3H-indol-3-ylidene)methyl]-2,4- dimethyl-lH-pyrrole-3-carboxylic acid (leq) in THF (15vol) was added l-ethyl-3-(3- dimethylaminopropyl)carbodumide hydrochloride (EDCHCl) (l.Seq),1-hydroxybenzotriazole (HOBT) (1.5eq) and TEA (2eq) and the solution was stirred at room temperature for 30 minutes. To this solution was added N,N-diemylethylenediamine (2eq) and the whole mass was stirred at room temperature for 8-10 hours. The reaction mass was then diluted with saturated sodium bicarbonate (8-lOvol) and the pH adjusted to pH 10 with the addition of a 50% NaOH aqueous solution (8-10vol). The whole mass was then extracted with ethyl acetate (3 x 5vol). The ethyl acetate layer was separated, dried over anhydrous sodium sulfate, then filtered. Evaporation of die ethyl acetate afforded the corresponding product. Molar yield = 70%. HPLC purity = 95.63%.IR (K-Br) cm4: 3276 (broad, N-H), 3063, 2966, 2925, 2807, 1675 (C=O), 1560, 1475, etc. 1H-NMR (DMSO-O6) 6 ppm: 0.97 (t, J=7.1Hz, 6H, 2 x -CH2-CH3), 2.42 (s, 3H, -CH3), 2.44 (s, 3H, -CH3), 2.47-2.56 (m, 6H, 3 x -N-CH2-), 3.25-3.31 (m, 2H3 -CO-NH-CH2-), 6.83-6,87 (m, IH, vinyl proton), 6.90-6.94 (t, J=5.9Hz, IH, aromatic ortho position), 7.43-7.47 (t, J=5.6Hz, IH, aromatic meta position), 7.74-7.78 (dd, J=5.9Hz, IH, aromatic ortho position), 7.72 (s, IH, amide NH, D2O exchangeable), 10.90 (s, IH, pyrrole NH, D2O exchangeable), 13.68 (s, IH, indole NH, D2O exchangeable).13C-NMR (DMSO-dfi) 6 ppm: 10.64 (1C, -CH3, DEPT), 11.92 (2C, 2 x -CH2-CH3, DEPT), 13.38 (1C, -CH3, DEPT), 37,02 (1C, -CH2-, DEPT), 46.55 (2C, 2 x -CH2-, DEPT), 51.69 (1C, -CH2-, DEPT), 105.90 (1C, d, aromatic ortho position, DEPT), 110.10 (1C, d, aromatic meta position, DEPT), 112.45 (1C, d, aromatic meta position, DEPT), 124,94 (1C, vinyl carbon, DEPT), 158.3 (1C, d, C-F, DEPT), 114.60 (bridge-head C of indole ring adjacent to >NH), 120.80, 134.50, 125.80, 136.70 (4C, pyrrole ring), 164.60 (1C, C=O),169.63 (IC5 C=O).Mass (m/z): (Nf+ 1) 399 (100%), [(M+2) +1] 401 (14%).
References:
WO2010/1167,2010,A2 Location in patent:Page/Page column 50-51

100-36-7
316 suppliers
$14.14/5gm:

557795-19-4
353 suppliers
inquiry
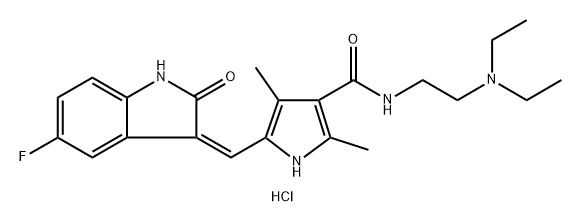
1327155-72-5
2 suppliers
inquiry

557795-19-4
353 suppliers
inquiry
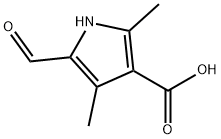
253870-02-9
370 suppliers
$6.00/1g

557795-19-4
353 suppliers
inquiry
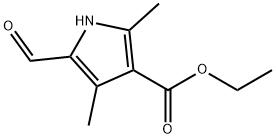
2199-59-9
240 suppliers
$8.00/250mg

557795-19-4
353 suppliers
inquiry