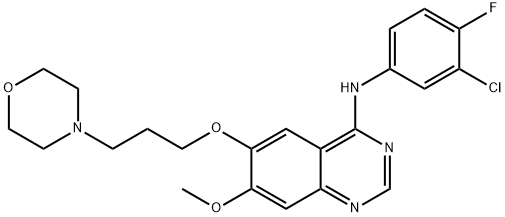
Gefitinib synthesis
- Product Name:Gefitinib
- CAS Number:184475-35-2
- Molecular formula:C22H24ClFN4O3
- Molecular Weight:446.9
367-21-5
512 suppliers
$5.00/25g
199327-59-8
35 suppliers
inquiry

184475-35-2
661 suppliers
$5.00/100mg
Yield:184475-35-2 99%
Reaction Conditions:
with potassium hydroxide in isopropyl alcohol at 20;
Steps:
1.e; 2.e (e) Synthesis of Gefitinib (F)
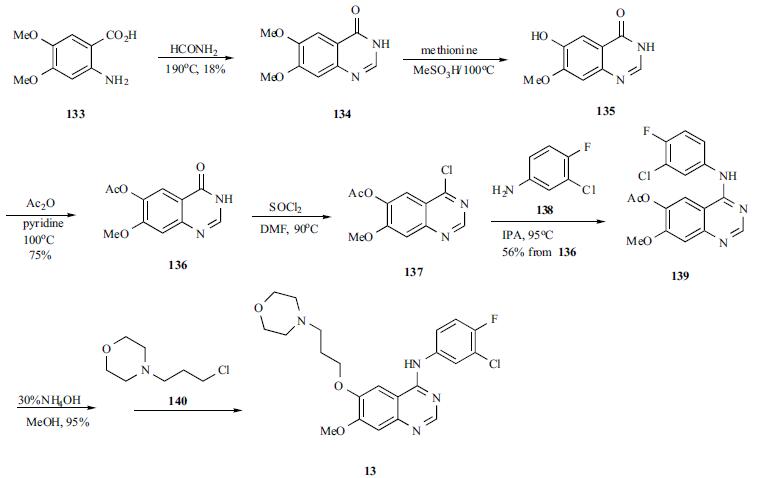
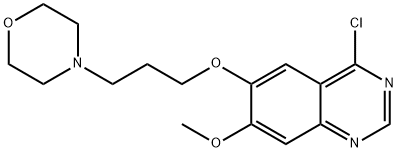
320g (0.98mol)7-methoxy-6- [3-morpholinepropoxy] -4-chloroquinazoline (E)Add to 400ml of isopropanol,Then 145 g (1 mol) of 3-chloro-4-fluoro-aniline was dissolved in 100 ml of isopropanol,It was added dropwise to the reaction solution and stirred at room temperature.After the reaction, 28 g (0.5 mol) of potassium hydroxide was added to the reaction solution, and the mixture was stirred at room temperature.Suction filtration, the filter cake was washed with an appropriate amount of isopropanol and ethanol in order, and dried under vacuum at 55 ° C to constant weight.Obtained a light beige powdery solid,That is 430 g of gefitinib, molar yield: 99%.
References:
CN110776471,2020,A Location in patent:Paragraph 0042; 0052-0053; 0054; 0064-0065
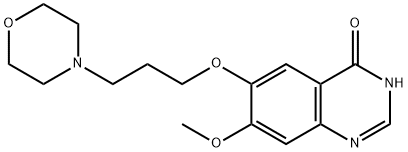
199327-61-2
273 suppliers
$6.00/1g

367-21-5
512 suppliers
$5.00/25g

184475-35-2
661 suppliers
$5.00/100mg
1018895-28-7
1 suppliers
inquiry
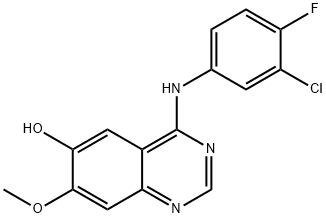
184475-71-6
225 suppliers
$5.00/250mg

184475-35-2
661 suppliers
$5.00/100mg
957621-67-9
0 suppliers
inquiry

184475-71-6
225 suppliers
$5.00/250mg

184475-35-2
661 suppliers
$5.00/100mg
125422-83-5
64 suppliers
$165.00/0.5g

184475-71-6
225 suppliers
$5.00/250mg

184475-35-2
661 suppliers
$5.00/100mg