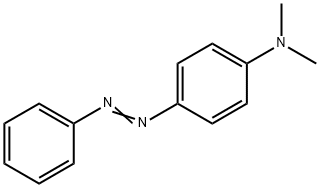
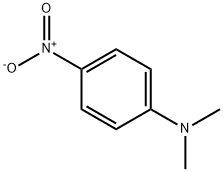
Solvent Yellow 2 synthesis
- Product Name:Solvent Yellow 2
- CAS Number:60-11-7
- Molecular formula:C14H15N3
- Molecular Weight:225.29
Yield:60-11-7 94%
Reaction Conditions:
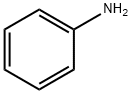
Stage #1:aniline with water;sodium nitrite in neat (no solvent) at 20;
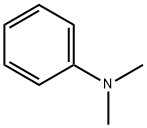
Stage #2:N,N-dimethyl-aniline in neat (no solvent) at 20; for 0.166667 h;
Steps:
2.2 Typical procedure for the preparation of N,N-dimethyl-4-(phenyldiazenyl)aniline
Aniline (2mmol, 0.1863g), nanomagnetic-supported sulfonic acid (γ-Fe2O3-SO3H) (1.3g), NaNO2 (4mmol, 0.276g) and 0.2mL of H2O were homogenized by grinding in a mortar with a pestle for a few minutes. The diazotization reaction lasted approximately 5-10min. Dimethylaminobenzene (2mmol, 0.2424g) was added to the diazonium salt and grinding continued for 10min. After completion of the reaction, the mixture was triturated with acetone (30mL). In the presence of a magnetic stirrer bar, catalyst moved on to the stirrer bar steadily and the reaction mixture turned clear within 10s. The catalyst was isolated by simple decantation. After evaporation of the solvent by rotary evaporator, the crude product was purified by recrystalization in EtOH/H2O, 9:1. N,N-dimethyl-4-(phenyldiazenyl)aniline was obtained in 94% yield (0.423g), mp 108-9°C, literature 107-9°C [28]. Recyclability of the catalyst was tested in this reaction, after completion of the reaction, the mixture was triturated with acetone and the catalyst was easily removed with an external magnet, after being washed with acetone and ethanol and dried at 70°C to be used in the next cycles. This process was repeated three times without significant loss of activity.
References:
Koukabi, Nadiya;Otokesh, Somayeh;Kolvari, Eskandar;Amoozadeh, Ali [Dyes and Pigments,2016,vol. 124,art. no. 4732,p. 12 - 17]
![1-[4-(dimethylamino)phenyl]propan-1-ol](/CAS/20180601/GIF/82946-80-3.gif)
