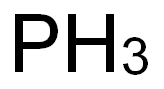
Phosphorus synthesis
- Product Name:Phosphorus
- CAS Number:7723-14-0
- Molecular formula:P
- Molecular Weight:30.97
Phosphorus exists in several allotropic forms: white (or yellow), red, and black (or violet). The last is of no industrial importance. Elemental yellow phosphorus extracted from bone was used to make “strike anywhere” matches. In 1845, the occupational disease “phossy jaw,” a jaw bone necrosis, was recognized in workers who manufactured such matches. A prohibitive tax imposed in 1912 on matches made from yellow phosphorus led to the use of less toxic materials, red phosphorus and phosphorus sesquisulfide. The United States appears to have lagged behind European countries in that signatories of the Berne Convention of 1906 agreed not to manufacture or import matches made with yellow phosphorus. Occasional injuries continued to result from using yellow phosphorus to manufacture fireworks until 1926, when an agreement was reached to discontinue using yellow phosphorus for this purpose.
The world production of elemental phosphorus exceeds 1,000,000 metric ton. It is manufactured either in electric or blast furnaces. Both depend on silica as a flux for the calcium present in the phosphate rock. Almost all of the phosphorus produced is converted into phosphoric acid or other phosphorus compounds.
Red phosphorus does not ignite spontaneously but may be ignited by friction, static electricity, heating, or oxidizing agents. Handling it in an aqueous solution helps prevent fires.
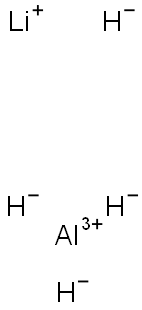
16853-85-3
412 suppliers
$14.00/1g
874483-75-7
0 suppliers
inquiry
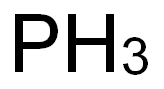
7723-14-0
223 suppliers
$40.39/1g

7803-51-2
3 suppliers
$1780.00/10g
Yield:7723-14-0 0%
Reaction Conditions:
in not given;byproducts: H2; reduction of PCl5 with LiAlH4; formation of decompn. products of PH5;;
References:
Wiberg, E.;Moedritzer, K. [Zeitschrift fur Naturforschung,1956,vol. 11,# B,p. 747 - 748]