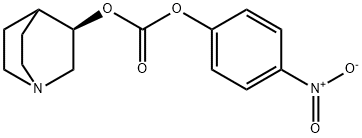
Solifenacin Impurity 1 HCl synthesis
- Product Name:Solifenacin Impurity 1 HCl
- CAS Number:1243274-81-8
- Molecular formula:C14H16N2O5
- Molecular Weight:292.29
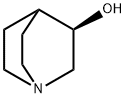
5070-13-3
240 suppliers
$5.00/5g
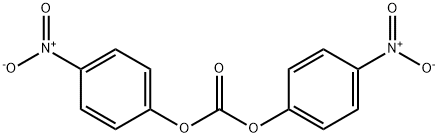
25333-42-0
307 suppliers
$60.00/1g

1243274-81-8
12 suppliers
inquiry
Yield:-
Reaction Conditions:
in N,N-dimethyl-formamide at 25 - 30;Product distribution / selectivity;Inert atmosphere;
Steps:
6
To a stirred solution of (3R)-quinuclidin-3-ol (Formula II, 100 gm) in dimethylformamide (400 ml), bis- (4-dinitrophenyl) carbonate (Formula III, 285.04 gm) was added with stirring at 25-30°C under nitrogen atmosphere. The reaction mass was stirred at 25-30°C for 2-3 hours. After completion of the reaction which was monitored by TLC, ( IS)-I -phenyl- 1, 2,3, 4-tetrahydroisoquinoline (Formula V, 171.44 gm) was added to the resultant brown colored reaction solution. The reaction mixture was further stirred at 25- 3O0C for 3-4 hrs. After completion of the reaction (by HPLC), the reaction solution was diluted with water (1000 ml) and the pH of the solution was adjusted to 1-2 using concentrated hydrochloric acid. The resulting reaction solution was extracted with diisopropylether (1000ml X 2) to separate the nitro-phenol. The aqueous layer was then mixed with dichloromethane (1000ml), the content was stirred, and dichloromethane layer was separated. Aqueous layer was re-extracted with dichloromethane (l OOOml).The combined dichloromethane was distilled off completely to obtain the residue. The residue was dissolved in water (1000 ml) and toluene (1000 ml) was added and the pH of the biphasic mixture was adjusted to 9-10 with ammonium hydroxide. The mixture was stirred and toluene layer was separated and aqueous layer was re-extracted with toluene (1000 ml). The combined toluene layer were washed with water (1000 ml) followed by solution of 0.5% sodium hydroxide (1000 ml X 2) and further washed with water (1000 ml). The toluene layer was distilled off completely to obtain the residue which was further dissolved in acetone (800 ml) and toluene 1080 ml). The solution was treated with Succininc acid (88.0 gm) and the mixture obtained was heated at 55-600C for 30 min. The mixture was further cooled to 10-150C, maintained for 60 min and filtered. The product was dried to afford Solifenacin Succinate (Formula VI) as white crystalline solid. Yield of the compound VI270 gm. HPLC purity : 99.85%: Chiral Purity: 99.99%
References:
WO2010/103529,2010,A1 Location in patent:Page/Page column 28-29