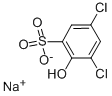
Sodium 3,5-chloro-6-hydroxybenzenesulfonate synthesis
- Product Name:Sodium 3,5-chloro-6-hydroxybenzenesulfonate
- CAS Number:54970-72-8
- Molecular formula:C6H3Cl2NaO4S
- Molecular Weight:265.05
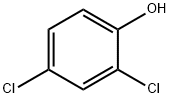
120-83-2
394 suppliers
$12.90/100g

54970-72-8
257 suppliers
$10.00/1g
Yield:54970-72-8 68%
Reaction Conditions:
with chlorosulfonic acid;thionyl chloride;sulfuric acid in dichloromethane at 0 - 40; for 12 h;Large scale;Reagent/catalyst;
Steps:
3 Example 3The preparation of sodium 3,5-dichloro-2-hydroxybenzenesulfonate was carried out according to the following procedure:
1) enamel reactor by adding 10kg / kg of dichloromethane, and then add 2.1kg / kg sulfonating agent, temperature control to -5-0 openStirring, step 1) sulfonating agent by weight ratio, containing sulfur trioxide 25% fuming sulfuric acid accounted for 40%, chlorosulfonic acidAccounting for 50%, the balance of thionyl chloride;2) adding 2, 4 dichlorophenol of 1 kg / kg in batches and controlling the temperature to below 0 DEG C during the adding process;3) After heating, the temperature was raised to 20 for 6h at 10 / h, 4h at 30 , 2h at 40 , TLCDetection of 2,4 - dichlorophenol raw materials reaction end;4) 2,4-Dichlorophenol After the reaction, the temperature was lowered to 0 ° C, and 15 kg / kg of purified water was added to the reaction vessel.Kg of 2-methyl tetrahydrofuran stirring 1h after standing still liquid;5) The aqueous layer was discarded and the organic layer was extracted with water and washed until the aqueous layer had a pH of 6-7;6) discard the water layer, the organic layer vacuum into the reactor, the temperature to 10-15 ;7) To the reaction kettle 3kg / kg saturated aqueous solution of sodium carbonate, stirring reaction at 100rpm for 0.5h;8) to stop stirring, standing still liquid, remove the water layer;9) The organic layer was concentrated under reduced pressure at 40 ° C to a volume reduction of one-half, and then 3 kg / kg of tetrahydrofuran was added thereto at 50 ° CUnder the concentrated concentration of the distillate until the gas phase detection of dichloromethane content of less than 2.0%, stop adding tetrahydrofuran, heated to 50° C;10) 50 under normal pressure to the reactor drop 5kg / kg of purified water, dropping to 3kg / kg, stop dropping, 50 PaulWarm stirring 3h, then 0.5h within the remaining 2kg / kg purified water;11) purified water drop is completed, heated to 60 ° C stirring 3h, and then 10 / h rate of cooling down to 30 ° C, the insulationStirring 3h, again to 10 / h heating rate to 60 temperature heating and stirring 3h, and finally to 10 / h rate of temperature dropWarm to 0 ° C after 2h stirring stirring;12) The filter cake is centrifuged until no filtrate flows out, then 8 kg / kg of organic solvent is added and stirred at 40 ° C for 3 h, then cooled to 10Deg. C to dryness and vacuum drying at 50 DEG C to constant weight to obtain sodium 3,5-dichloro-o-hydroxybenzenesulfonate, the organic solvent is methyl tert-butylButyl ether and n-heptane mixed solvent, in which the weight of methyl tert-butyl ether 60%, n-heptane weight 40%.2, 4 dichlorophenol feeding 2kg, 3,5-dichloro-2-hydroxybenzene sulfonic acid sodium receiving 2.20kg, the yield was 68%, takeThe purity was 99.4% by HPLC and the particle size range was 30-50 microns.
References:
CN105906533,2016,A Location in patent:Paragraph 0019
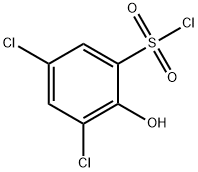
23378-88-3
61 suppliers
$14.14/250mgs:

54970-72-8
257 suppliers
$10.00/1g

23378-88-3
61 suppliers
$14.14/250mgs:
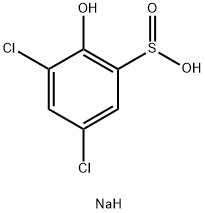
125927-96-0
0 suppliers
inquiry

54970-72-8
257 suppliers
$10.00/1g