
Sodium 3-nitrobenzenesulphonate synthesis
- Product Name:Sodium 3-nitrobenzenesulphonate
- CAS Number:127-68-4
- Molecular formula:C6H4NNaO5S
- Molecular Weight:225.15
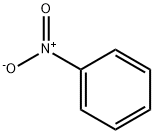
98-95-3
15 suppliers
$14.00/25g

127-68-4
536 suppliers
$5.00/25g
Yield:127-68-4 98.4%
Reaction Conditions:
Stage #1: nitrobenzenewith chlorosulfonic acid at 120; for 5 h;
Stage #2: with sodium carbonate in water at 20;Reagent/catalyst;
Steps:
2.1; 2.2 Example 2
1) Add 246g (2.0mol) of nitrobenzene and 139g (1.2mol) of chlorosulfonic acid to a 500mL four-necked flask equipped with a thermometer, mechanical agitation and exhaust gas absorption device, stir and raise the temperature to 120 °C.The sulfonation reaction was carried out for 3 hours.The hydrogen chloride gas generated during the reaction is introduced into 200 mL of deionized water through a tail gas absorption device, and finally obtained in a hydrogen chloride aqueous solution.The mass concentration of hydrogen chloride was 17.5%.2), after the end of the above reaction, cool to room temperature,Add 500 mL of water and 63.6 g (0.6 mol) of sodium carbonate and stir.Allow to stand and make the oil moisture layer.Among them, the oil layer is washed three times with water (500mL × 3),The mixture was dried with 35 g of molecular sieve to obtain 95 g of a nitrobenzene recovery liquid.The content is 99.3%.The aqueous layer solution is dried by a spray drying device (atomization speed 15-20 mL/min, inlet temperature 300 °C, outlet temperature 100 °C),266 g of sodium nitrobenzenesulfonate was obtained as a dry product, and the content was 99.0%.The yield was 98.4% (based on the consumption of nitrobenzene).
References:
CN108997175,2018,A Location in patent:Paragraph 0046-0059; 0062-0066