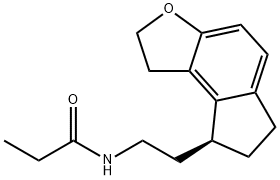
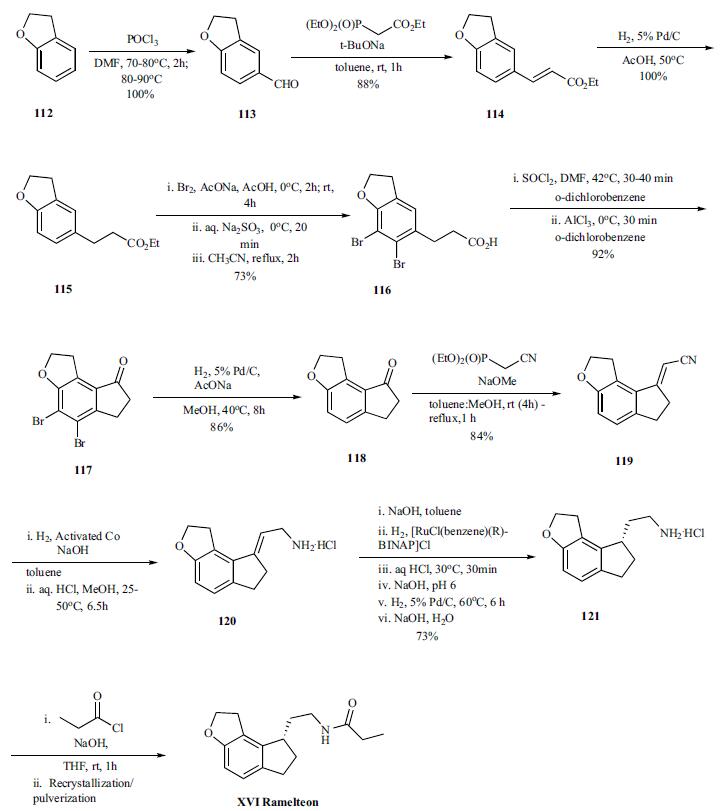
Ramelteon synthesis
- Product Name:Ramelteon
- CAS Number:196597-26-9
- Molecular formula:C16H21NO2
- Molecular Weight:259.34
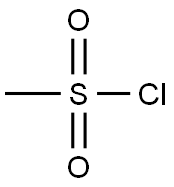
124-63-0
6 suppliers
$10.00/5g
![(S)-N-[2-[2,3-Dihydro-6-hydroxy-7-(2-hydroxyethyl)-1H-inden-1-yl]ethyl]propanamide](/CAS2/GIF/196597-88-3.gif)
196597-88-3
10 suppliers
$80.00/1mg

196597-26-9
366 suppliers
$9.00/1mg
Yield:196597-26-9 86%
Reaction Conditions:
Stage #1: methanesulfonyl chloride;(S)-N-[2-(6-hydroxy-7-(2-hydroxyethyl)-2,3-dihydro-1H-inden-1-yl)ethyl]propionamidewith pyridine at -10 - -5; for 0.833333 h;
Stage #2: with triethylamine in ethyl acetate;Heating;Further stages.;
References:
Uchikawa, Osamu;Fukatsu, Kohji;Tokunoh, Ryosuke;Kawada, Mitsuru;Matsumoto, Kiyoharu;Imai, Yumi;Hinuma, Shuji;Kato, Koki;Nishikawa, Hisao;Hirai, Keisuke;Miyamoto, Masaomi;Ohkawa, Shigenori [Journal of Medicinal Chemistry,2002,vol. 45,# 19,p. 4222 - 4239]
![(S)-2-(2,6,7,8-tetrahydro-1H-indeno[5,4-b]furan-8-yl)acetonitrile](/CAS/GIF/1185516-79-3.gif)
1185516-79-3
20 suppliers
inquiry

123-62-6
322 suppliers
$17.00/25g

196597-26-9
366 suppliers
$9.00/1mg
![(S)-2-(2,6,7,8-tetrahydro-1H-indeno[5,4-b]furan-8-yl)acetonitrile](/CAS/GIF/1185516-79-3.gif)
1185516-79-3
20 suppliers
inquiry

196597-26-9
366 suppliers
$9.00/1mg
![(S)-N-[2-[2,3-Dihydro-6-hydroxy-7-(2-hydroxyethyl)-1H-inden-1-yl]ethyl]propanamide](/CAS2/GIF/196597-88-3.gif)
196597-88-3
10 suppliers
$80.00/1mg

196597-26-9
366 suppliers
$9.00/1mg
![1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-78-1.gif)
196597-78-1
262 suppliers
$31.00/250mg

196597-26-9
366 suppliers
$9.00/1mg