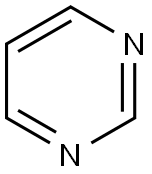
Pyrimidine synthesis
- Product Name:Pyrimidine
- CAS Number:289-95-2
- Molecular formula:C4H4N2
- Molecular Weight:80.09
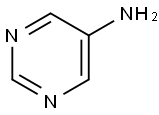
591-55-9
201 suppliers
$6.00/250mg

289-95-2
316 suppliers
$15.00/1g
Yield:289-95-2 55%
Reaction Conditions:
with isopentyl nitrite in tetrahydrofuran at 120; under 5250.53 Torr; for 0.333333 h;
Steps:
4.6. General Procedure for Hydrodeazoniation of Substrates
General procedure: A solution of the selected heterocyclic starting material (1.00 mmol) in THF (10 mL) and a solutionof isopentyl nitrite (141 mg, 1.20 mmol) in THF (10 mL) were both pumped at a flow rate of 0.25 mLmin1 with a Vapourtech ‘Easy MedChem V3’ system, meeting at a PTFE T-piece and the outputflowing through a 10.0 mL coil reactor maintained at 120 °C, giving a residence time of 20 min.The pressure of the system was maintained at 7 bar with a back-pressure regulator. For compoundswhere an isolated yield was reported: the output mixture was concentrated under reduced pressureto give an oil (or powder). The oil (or powder) was purified using column chromatography withvarious mixtures of ethyl acetate and hexane as the eluent, or by recrystallisation using methanol, togive isolated compounds that showed no impurities by NMR spectroscopy. For compounds where aconversion was reported (due to volatility of products), the output mixture was carefully concentratedunder a reduced pressure of 100 mbar for 10 min and the conversion was calculated by integration ofproduct peaks to a quantified internal standard (nitrobenzene).
References:
R?der, Liesa;Nicholls, Alexander J.;Baxendale, Ian R. [Molecules,2019,vol. 24,# 10,art. no. 1996]
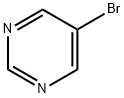
4595-59-9
460 suppliers
$6.00/1g

289-95-2
316 suppliers
$15.00/1g
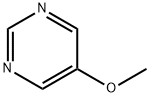
31458-33-0
94 suppliers
$26.00/100mg
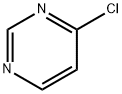
17180-93-7
145 suppliers
$98.00/1g

289-95-2
316 suppliers
$15.00/1g
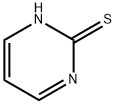
1450-85-7
233 suppliers
$15.00/5g

289-95-2
316 suppliers
$15.00/1g
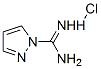
4023-02-3
276 suppliers
$5.00/5g

289-95-2
316 suppliers
$15.00/1g