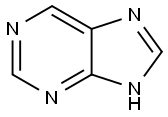
Purine synthesis
- Product Name:Purine
- CAS Number:120-73-0
- Molecular formula:C5H4N4
- Molecular Weight:120.11
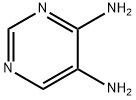
13754-19-3
197 suppliers
$45.00/250mg
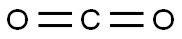
124-38-9
130 suppliers
$214.00/14L

120-73-0
159 suppliers
$9.00/100mg
Yield:-
Reaction Conditions:
with N,N′-bis(2,6-diisopropylphenyl)imidazol-2-ylidene hydrochloride;phenylsilane in tetrahydrofuran at 70; under 750.075 - 2250.23 Torr; for 24 h;Schlenk technique;Inert atmosphere;Glovebox;
Steps:
1.A A-Synthesis of Benzimidazole and Derivatives from Aromatic 1,2-Diamines
General procedure: A-Synthesis of Benzimidazole and Derivatives from Aromatic 1,2-Diamines
The first results presented describe the synthesis of benzimidazole rings and derivatives from aromatic 1,2-diamines. In this case, the amine R1NH2 and the nitrogenous nucleophilic agent R5R6NH are two reactive functional groups of one and the same molecule (diamine) and are thus connected via a covalent bond. This bond is preferably an aromatic ring of benzene, pyridine or pyrimidine type and the ring formed during the reaction is a nitrogenous heterocycle comprising 5 atoms of imidazole type. In the case where the nucleophile is oxygen-based (R7OH), the rings obtained are benzoxazoles. The results presented were produced by preferably using two sources of different reducing agents, polymethylhydrosiloxane (PMHS), sold by Aldrich under the reference 176206, and phenylsilane (PhSiH3), sold by Aldrich. In the case of the PMHS, as a silane is a polymer, the number of equivalents introduced is given with respect to the number of hydrides introduced and thus the number of monomers introduced with respect to the amine. Thus, the introduction of 3 equivalents of PMHS corresponds to the introduction of 3 equivalents of hydride and thus 3 equivalents of monomers of the PMHS with respect to the amine. Different (pre)catalysts were tested for the reaction.
References:
US2015/148535,2015,A1 Location in patent:Paragraph 0.192; 0193
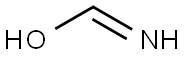
60100-09-6
0 suppliers
inquiry
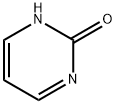
557-01-7
221 suppliers
$26.00/1g
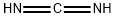
151-51-9
46 suppliers
inquiry

120-73-0
159 suppliers
$9.00/100mg

60100-09-6
0 suppliers
inquiry
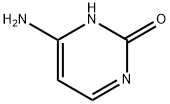
71-30-7
728 suppliers
$5.00/10g

120-73-0
159 suppliers
$9.00/100mg
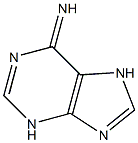
134461-75-9
1 suppliers
inquiry