PHENYL TRIFLUOROACETATE synthesis
- Product Name:PHENYL TRIFLUOROACETATE
- CAS Number:500-73-2
- Molecular formula:C8H5F3O2
- Molecular Weight:190.12
![[BIS(TRIFLUOROACETOXY)IODO]PENTAFLUOROBENZENE](/CAS/GIF/14353-88-9.gif)
14353-88-9
76 suppliers
$19.00/100mg
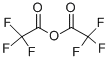
76-05-1
680 suppliers
$9.00/1g

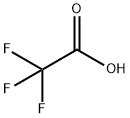
71-43-2
694 suppliers
$11.19/25ML

500-73-2
102 suppliers
$10.00/1g
Yield:-
Reaction Conditions:
with trifluoroacetic anhydride at 100; for 3 h;Temperature;
Steps:
Heating a 100 mM solution of C6F5-Iin(TFA)2 at 150°C for 3 hrs in 100 mM TFAA/HTFA under 500 psi of methane or ethane (125 psi for PrH) was shown by the 1H-NMR of the crude reaction mixtures to generate the respective trifluoroacetate (TFA) mono-esters and 1 ,2-TFA-diesters (for EtH and PrH) as shown in Eq. 9: R-H + C6F5-l'"(TFA)2 R-TFA + C6F5-l' + HTFA TFAA HTFA (R = Me, Et, Pr, Ph) gq 9 1361.193W01 / TSRI Case 1560.1 PCT / FLA0129P Table 4. Conversions of Unactivated Hydrocarbons with Iodine, Selenium, and Tellurium Reagents The reactions are highly selective and no hydrocarbon derived products are observed besides the respective mono- and 1,2-di-esters (Table 5). 19F-NMR analysis of the solutions post-reaction showed that C6F5-Ini(TFA)2 is converted to C6F5-I1, as the reaction proceeds with no intermediate iodine species observed.
References:
THE SCRIPS RESEARCH INSTITUTE;PERIANA, Roy;HASHIGUCHI, Brian G.;KONNICK, Michael M.;BISCHOF, Steven M. WO2014/130987, 2014, A1 Location in patent:Page/Page column 30; 31
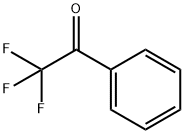
434-45-7
284 suppliers
$12.00/5g

500-73-2
102 suppliers
$10.00/1g

96254-05-6
10 suppliers
$208.00/10ml
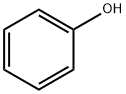
108-95-2
737 suppliers
$14.00/25g

500-73-2
102 suppliers
$10.00/1g
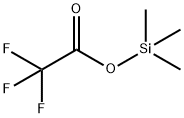
400-53-3
118 suppliers
$7.00/5g
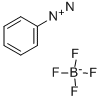
369-57-3
39 suppliers
inquiry
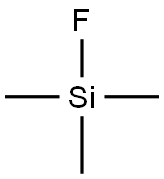
420-56-4
71 suppliers
$95.00/10 g

350-70-9
9 suppliers
$159.00/25ml

500-73-2
102 suppliers
$10.00/1g