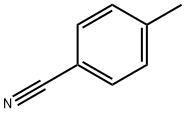
p-Tolunitrile synthesis
- Product Name:p-Tolunitrile
- CAS Number:104-85-8
- Molecular formula:C8H7N
- Molecular Weight:117.15
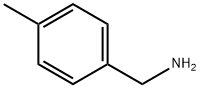
104-84-7
222 suppliers
$21.00/5g

104-85-8
323 suppliers
$11.00/25g
Yield:104-85-8 98%
Reaction Conditions:
with C22H22Cl2FeN2O8(2-)*2C16H36N(1+);oxygen in neat (no solvent) at 100; under 760.051 Torr; for 0.5 h;Green chemistry;
Steps:
Typical experimental procedure for aerobic oxidation of amine
General procedure: A double necked round bottomed flask (10 ml), equipped with magnetic stirrer was charged with amine (1 mmol) and catalyst 3 (5 wt %) and the resulting mixture was heated at 100 °C for 0.5 h under constant purging of molecular oxygen. After completion of the reaction, the mixture was diluted with ethyl acetate and the recovered catalyst was separated by decantation. The combined organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the crude product, which was further purified by column chromatography over silica gel using a solvent system of hexane and ethyl acetate (4:1) as eluent.
References:
Varyani, Manish;Khatri, Praveen K.;Jain, Suman L. [Tetrahedron Letters,2016,vol. 57,# 7,p. 723 - 727]

106-42-3
411 suppliers
$19.00/25ML

104-85-8
323 suppliers
$11.00/25g
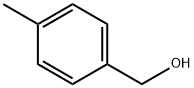
589-18-4
310 suppliers
$5.00/5g

104-85-8
323 suppliers
$11.00/25g
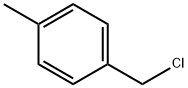
104-82-5
363 suppliers
$10.00/5mL

104-85-8
323 suppliers
$11.00/25g
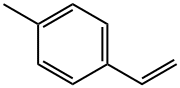
622-97-9
136 suppliers
$10.00/5mL

104-85-8
323 suppliers
$11.00/25g