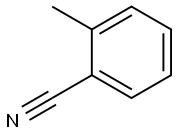
o-Tolunitrile synthesis
- Product Name:o-Tolunitrile
- CAS Number:529-19-1
- Molecular formula:C8H7N
- Molecular Weight:117.15

529-20-4
330 suppliers
$5.00/5g

529-19-1
250 suppliers
$17.89/50g
Yield:529-19-1 92%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;sodium perchlorate;acetic acid;lithium hexamethyldisilazane in acetonitrile; for 12 h;Electrochemical reaction;
Steps:
General procedure: Preparative electrolysis experiments were performed using 263 APotentiostat/Galvanostat (Princeton Applied Research, USA). 0.1 MNaClO4-CH3CN solution (10 mL) containing aldehydes (1 mmol),TEMPO (0.1 mmol), HMDS (2.5mmol) and AcOH (2.5mmol) was electrolyzedwith stirring in an undivided cell (30 mL) equipped with twoplatinum sheets as anode (1.5 cm2) and cathode (3.0 cm2) respectivelyat a constant potential of 1.5 V vs Ag/Ag+ (0.1MAgNO3 in acetonitrile).The electrode separation was 1 cm. When the reaction was finished,10mL of saturatedNa2SO3 solution was added into the reactionmixtureand stirred for 15 min. Then the mixture was extracted with CH2Cl2(20 mL × 3). The organic layer was dried with anhydrous Na2SO4 andconcentrated in a rotary evaporator. The productswere obtained via purificationof column chromatography and their structures were confirmedby 1H NMR, 13C NMR and MS. NMR was performed on a BrukerAvance III spectrometer. GC-MS was performed on the Thermo TraceISQ instrument with TG 5MS capillary column.
References:
Chen, Qiguo;Fang, Chaojie;Shen, Zhenlu;Li, Meichao [Electrochemistry Communications,2016,vol. 64,p. 51 - 55]
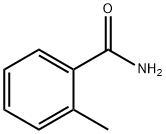
527-85-5
70 suppliers
$29.00/25g

529-19-1
250 suppliers
$17.89/50g

89-95-2
203 suppliers
$22.00/10g

529-19-1
250 suppliers
$17.89/50g
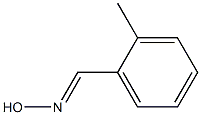
14683-79-5
3 suppliers
inquiry

529-19-1
250 suppliers
$17.89/50g
122382-54-1
0 suppliers
inquiry

529-19-1
250 suppliers
$17.89/50g