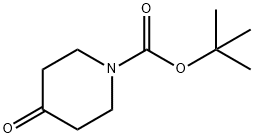
N-(tert-Butoxycarbonyl)-4-piperidone synthesis
- Product Name:N-(tert-Butoxycarbonyl)-4-piperidone
- CAS Number:79099-07-3
- Molecular formula:C10H17NO3
- Molecular Weight:199.25
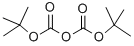
24424-99-5
839 suppliers
$13.50/25G
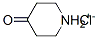
41979-39-9
116 suppliers
$15.00/10mg

79099-07-3
543 suppliers
$5.00/5g
Yield:79099-07-3 100%
Reaction Conditions:
Stage #1: di-tert-butyl dicarbonate;4-piperidone hydrochloridewith triethylamine;dmap in methanol at 20; for 20 h;
Stage #2: with hydrogenchloride in dichloromethane;
Steps:
8
Triethylamine (19.2g, 190mmol) was added to a stirring solution of 4-piperidone monohydrate hydrochloride (20.0g, 131mmol) in methanol (300mL) and stirred for 5min. Boc20 (34g, 168mmol) was added in portions over a 5min period, followed by DMAP (0.4g, 3mmol). The solution was stirred at ambient temperature for 20h. The methanol was removed under reduced pressure and the crude was dissolved in dichloromethane (100mL). The organic phase was washed with HCI (2M, 2 x 70mL) sat. Na2C03 (70mL) and sat NaCI (50mL), dried over Na2S04, filtered and evaporated to dryness to yield 1- Boc-4-piperidone as a white solid in quantitative yield. 1H NMR (CDCI 3400m Hz) δ 3.71 (t, J=6.2Hz, 4H), 2.44 (t, J=6.2Hz, 4H), 1.49 (s, 9H).
References:
WO2012/142668,2012,A1 Location in patent:Page/Page column 50; 51
109384-19-2
478 suppliers
$5.00/1g

79099-07-3
543 suppliers
$5.00/5g

41661-47-6
93 suppliers
$45.00/250mg

24424-99-5
839 suppliers
$13.50/25G

79099-07-3
543 suppliers
$5.00/5g

24424-99-5
839 suppliers
$13.50/25G
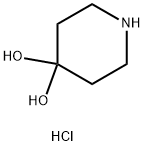
40064-34-4
366 suppliers
$10.00/5g

79099-07-3
543 suppliers
$5.00/5g

24424-99-5
839 suppliers
$13.50/25G

40064-34-4
366 suppliers
$10.00/5g

79099-07-3
543 suppliers
$5.00/5g