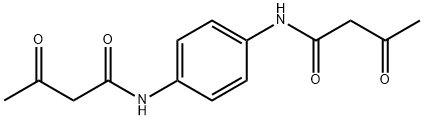
N,N'-(1,4-Phenylene)bis(acetoacetamide) synthesis
- Product Name:N,N'-(1,4-Phenylene)bis(acetoacetamide)
- CAS Number:24731-73-5
- Molecular formula:C14H16N2O4
- Molecular Weight:276.29
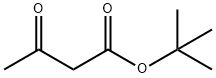
1694-31-1
364 suppliers
$6.00/25g
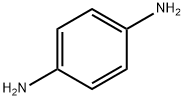
106-50-3
613 suppliers
$19.00/25g

24731-73-5
110 suppliers
$75.00/1g
Yield: 50%
Reaction Conditions:
Stage #1:tert-butyl acetoacetate in 5,5-dimethyl-1,3-cyclohexadiene at 140; for 0.166667 h;
Stage #2:1,4-phenylenediamine in 5,5-dimethyl-1,3-cyclohexadiene at 140; for 1.16 h;
Steps:
6.2 Synthesis of N,N'-(1,4-phenylene)bis(3-oxobutanamide) (Phenylene diam AA) (Scheme 11)
Synthesis of N,N'-(1,4-phenylene)bis(3-oxobutanamide) (Phenylene diam AA) (Scheme 11)
A solution of tert-butyl acetoacetate (21.0 mmol, 3.38 g) in xylenes (50 mL) was heated to 140° C. (internal temperature) under magnetic stirring.
After 10 minutes, a solution of 1,4-phenylene diamine (10 mmol) in xylenes (100 mL) was added dropwise using an addition funnel over a time course of 10 min.
After addition, stirring was continued for 1 hour at 140° C. (internal temperature).
Afterwards, the bulk xylenes were removed in vacuo affording a pale yellow precipitate.
The solids were then redissolved in ethyl acetate (50 mL) and hexane was added dropwise until the solution started to become cloudy.
At this point, the solution was left at room temperature for a minimum of 12 hours.
The formed white solids were filtered off and dried in vacuo (40° C.) yielding the pure bisacetoacetamide.
Yield: 50%, 1H NMR [(DMSO-d6), 300 MHz]: 10.80 (s, 2H, NH), 7.50 (s, 4H, phenyl protons), 3.55 (s, 4H, COCH2CO), 2.22 (s, 6H, COCH3).
References:
UNIVERSITEIT GENT;ECOLE SUPéRIEURE DE PHYSIQUE ET DE CHIMIE NDUSTRIELLES LA VILLE DE LA VILLE DE PARIS;CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE - CNRS;DU PREZ, Filip;WINNE, Johan;DENISSEN, Wim;LEIBLER, Ludwik;NICOLA?, Renaud US2017/327625, 2017, A1 Location in patent:Paragraph 0599; 0600; 0601

141-97-9
788 suppliers
$5.00/25g

106-50-3
613 suppliers
$19.00/25g

24731-73-5
110 suppliers
$75.00/1g