Mebendazole synthesis
- Product Name:Mebendazole
- CAS Number:31431-39-7
- Molecular formula:C16H13N3O3
- Molecular Weight:295.29
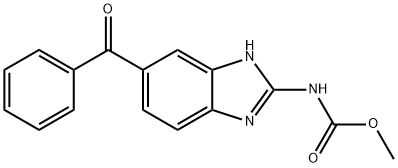
39070-63-8
172 suppliers
$26.00/5g
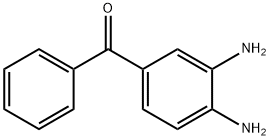
40943-37-1
16 suppliers
inquiry

31431-39-7
585 suppliers
$5.00/100mg
Yield:31431-39-7 95%
Reaction Conditions:
with acetic acid in toluene
Steps:
1-3 Comparative Example 1
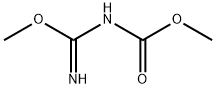
Synthesis of Form C Toluamisole by Traditional Technology:In a 500 ml four-necked flask, 50 g of 3,4-diaminobenzophenone was added.O-methylisourea formic acid methyl acetate toluene solution 32.8g (13%, pure)32.5g of acetic acid, warmed to 40C (35-45C), heat-retained for 16 hours (16-17 hours). 70g of formic acid was added, heated to reflux, temperature was 80C (78-85C), refluxed for 6 hours (6-7 hours) ? Reduce the temperature to 65C (60-70C), add 85ml of methanol, and heat and stir at 65C (60-70C) for 1 hour (1-2 hours). Cool down to 35C (30-40C). It was filtered, washed with 50 ml of methanol, and the filter cake was dried at 100 C (90-110 C) for 24 hours to obtain 62.6 g of a crude product with a yield of 90%.In a 500 ml four-necked flask, add 30 g of crude tomidazole, 125 g of formic acid, and 0.6 g of activated carbon. Stir, heat to 65 ° C (60-70 ° C), keep at least 1 hour, and heat filter. The filtrate was transferred to a 500-ml four-necked flask, and the temperature was lowered to 50 ° C (40-60 ° C). 100 ml of water for crystallization was added once, and 0.5 g of tomidazole C crystal seed was added. Turn down the stirring speed, lower the temperature to 40C (35-45C), and keep it for 1 to 2 hours. Add 260ml of secondary crystallization water, continue to lower the temperature to 20 ° C (15-25C), and keep it for 1 to 2 hours. Filtered, washed with 360ml purified water, and the filter cake was dried at 90 ° C (80-100C) to obtain 29g of C-type tomidimazole with a yield of 95%. Comparative Example 2In a 500ml four-necked flask, add 50g of crude tomidazole and 250ml of methanol, stir, heat to 55C (50-60C), dropwise add 16g of sulfuric acid, dissolve the material, add 1.0g of activated carbon, keep at least 1 hour, and heat filter.The filtrate was transferred to a 500 ml four-necked flask, and the temperature was lowered to 50 ° C (40-60C), and 1 g of crystal C-formimidazole seed crystals were added and kept for 1 hour. The temperature was lowered to 40 ° C (35-45C), 200 ml of water was added, 100 g of 15% sodium hydroxide was added dropwise, the pH was adjusted to 6, and the temperature was maintained for 1 to 2 hours. Filter, 300mlIt was washed with purified water, and the filter cake was dried at 90 ° C (80-100C) to obtain 48.5 g of toluzazole in the C crystal form with a yield of 95%. In this comparative example, the crystal form conversion in methanolic sulfuric acid was adopted, and the yield of the toluzazole in the crystal form C was significantly lower than that in methanol nitrate.Comparative Example 3In a 500ml four-necked flask, add 50g of crude tomidazole and 250ml of methanol, stir, heat to 55C (50-60C), dropwise add 16g of sulfuric acid, dissolve the material, add 1.0g of activated carbon, keep at least 1 hour, and heat filter.The filtrate was transferred to a 500 ml four-necked flask, and the temperature was lowered to 50 ° C (40-60C), and 1 g of crystal C-formimidazole seed crystals were added and kept for 1 hour. The temperature was lowered to 40 ° C (35-45C), 200 ml of methanol was added, 100 g of 15% sodium hydroxide was added dropwise, the pH was adjusted to 6, and the temperature was maintained for 1 to 2 hours.Filtration, washing with 300 ml of purified water, drying of the filter cake at 90 ° C (80-100C), 49 g of A-type tomidazole were obtained with a yield of 96% and 100% of A-type. In the crystallization process of this comparative example, the addition of methanol instead of water could not achieve the correct conversion of the crystal form, and only Form A tolumidazole was obtained, and Form C Toluidazole was not obtained.
References:
Lianyungang Yahui Pharmaceutical And Chemical Co., Ltd.;Zhang Jianfeng;Zhu Yunbing;Liu Yonglin;He Xiaowei;Huang Jinzhan;Li Zhiling;Zhang Wentao CN110283128, 2019, A Location in patent:Paragraph 0043-0050
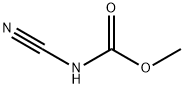
21729-98-6
45 suppliers
inquiry

39070-63-8
172 suppliers
$26.00/5g

31431-39-7
585 suppliers
$5.00/100mg
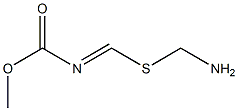
39259-32-0
5 suppliers
inquiry

39070-63-8
172 suppliers
$26.00/5g

31431-39-7
585 suppliers
$5.00/100mg
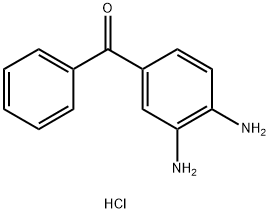
57070-71-0
21 suppliers
inquiry

31431-39-7
585 suppliers
$5.00/100mg
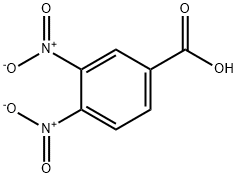
528-45-0
162 suppliers
$10.00/1g

31431-39-7
585 suppliers
$5.00/100mg