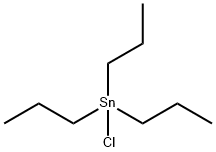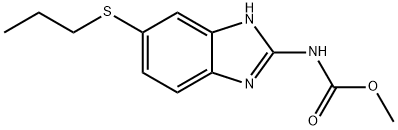
Albendazole synthesis
- Product Name:Albendazole
- CAS Number:54965-21-8
- Molecular formula:C12H15N3O2S
- Molecular Weight:265.33

Yield:54965-21-8 520 kg
Reaction Conditions:
with hydrogenchloride in water;acetone at 85;Large scale;
Steps:
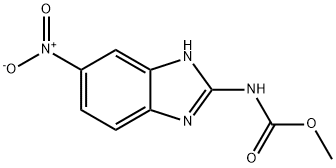
1.f (f) Preparation of Albendazole
(f)
Preparation of Albendazole
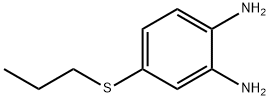
4-Propylthio-o-phenylenediamine (400 kg) was treated with acetone (400 L).
Then water (380 L) and conc. HCl (360 kg) was added to it.
Exothermic reaction observed upto 48° C.
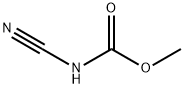
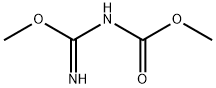
Reaction mass was cooled to room temperature and methyl-N-Cyano Carbamate was added.
The reaction mass was heated to 80-85° C.
The pH was adjusted to 4-4.5 by concentrated HCl and centrifuged.
The material and washed with hot water, tap water, methanol and finally with acetone.
Weight: 500-520 kg.
References:
US2013/303782,2013,A1 Location in patent:Paragraph 0031
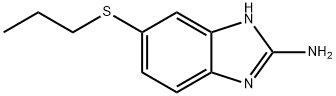
80983-36-4
81 suppliers
$185.00/50mg

79-22-1
2 suppliers
$38.00/1000g

54965-21-8
920 suppliers
$16.80/1G