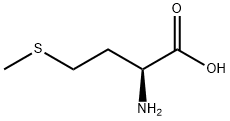
L-Methionine synthesis
- Product Name:L-Methionine
- CAS Number:63-68-3
- Molecular formula:C5H11NO2S
- Molecular Weight:149.21
59-51-8
823 suppliers
$5.00/100mg

63-68-3
906 suppliers
$5.00/25g
Yield:63-68-3 33%
Reaction Conditions:
Stage #1: DL-methionine in water at 0.99; for 96 h;
Stage #2: in water at 59.99;Purification / work up;
Steps:
1; 2
A physical mixture of crystals of the racemic compound (DL-methionine) and the L-enantiomer (387.44 g and 59.69 g) were added to a 2000 ml reactor vessel. The composition was chosen to represent a possible output of a previous partial enrichment step via another reaction or separation step. This initial mixture was only slightly enriched by the target enantiomer (optical purity 56.7%). 1000 g of water were added and the slurry was properly agitated and kept at isothermal conditions at 274.15 K for 4 days to ensure thermodynamic solid/liquid phase equilibrium. The duration can probably be shortened much in terms of process i.e. method optimization. Analysis by means of chiral chromatography yielded an optical purity in the liquid phase of 93.8% L-enantiomer after this period. Step 2:Subsequently the solid phase was filtrated off and the liquid phase was decanted to another reactor vessel and heated to a temperature of 333.15 K.A fraction of the solvent was evaporated by means of vacuum distillation at 190 mbar aiming to enter the 2-phase area defined by the solubility isotherm at this temperature. The eutectic composition of the methionine enantiomers in water at a temperature of 333.15 K was experimentally determined earlier to 86% optical purity. During the partial removal of the solvent and the generation of supersaturation spontaneous nucleation occurred and the L-enantiomer crystallized with 100% optical purity within the reactor vessel. The final crystalline product mass after solvent removal exhibited an optical purity of 98.6%. The maximal theoretical yield for this system under the conditions of the experiment, governed by the thermo-dynamic equilibrium, is obtained at the phase boundary between the 2- and the 3-phase area (-54% for this case). For this experimental run, the second process step was stopped clearly above the inner phase boundary. An overall yield of 33% (19.72 g product L-methionine/59.69 g) was obtained which was also diminished by product losses during the two filtration steps, which leaves potential for process improvements. By complete avoidance of crystallization of the mother liquor onto the crystalline product, 100% purity can be achieved. Promising techniques for this issue are already commercially available and not part of the present application. The mother liquor, which was filtrated off, showed an optical purity of 86.0%. That makes this phase suitable to be used in combination with new feed material in order to enhance further the method efficiency. Since this solution has to be diluted again for reuse in the above-described step 1, an even less optical enriched output stream from a chromatographic separation could be used for mixing in favourable synergistic manner.
References:
US2011/263896,2011,A1 Location in patent:Page/Page column 3
348-67-4
385 suppliers
$5.00/1g

63-68-3
906 suppliers
$5.00/25g
2488-15-5
289 suppliers
$10.00/1g

63-68-3
906 suppliers
$5.00/25g
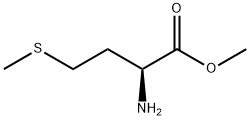
10332-17-9
21 suppliers
inquiry

63-68-3
906 suppliers
$5.00/25g
![(S)-2-[{(allyloxy)carbonyl}amino]-4-(methylthio)butanoic acid](/CAS/20200401/GIF/90508-21-7.gif)
90508-21-7
1 suppliers
inquiry

63-68-3
906 suppliers
$5.00/25g