Ethyl chloroacetate synthesis
- Product Name:Ethyl chloroacetate
- CAS Number:105-39-5
- Molecular formula:C4H7ClO2
- Molecular Weight:122.55
ClCH2COOH+C2H5OH[H2SO4]→ClCH2COOC2H5+H2O
Reaction: Add chloroacetic acid, ethanol and benzene into the esterification pot, turn on the stirrer, slowly add sulfuric acid, heat to reflux, continuously steam out the benzene-water azeotrope, and de-esterify the water generated by condensation and separator layering, Benzene is refluxed into the esterification pot, cooled and discharged when there is no more water to steam out, the crude ester is washed with saturated sodium bicarbonate solution and water to neutrality, dried with anhydrous calcium chloride, distilled, and collected The fraction at 144-146°C is the finished product of ethyl chloroacetate, the content is ≥99.0%, and the yield is 85%.
Yield:105-39-5 97.4%
Reaction Conditions:
at 105; for 3 h;Molecular sieve;Temperature;
Steps:
1-3
A method for synthesizing betaine hydrochloride, wherein 94.5 g of monochloroacetic acid, 12 g of a catalyst, and 59 ethanol are sequentially added to a reaction flask.Then, the temperature was raised at 105 ° C, the molecular sieve was dehydrated at reflux, and the reaction was completed in 3 hours. The reaction solution was washed twice with water, and the separated ethyl chloroacetate was distilled under reduced pressure. The ethyl acetate ethyl acetate fraction was 72 ° C / 0.09 kPa. %, yield 97.4%.
References:
CN109942445,2019,A Location in patent:Paragraph 0008; 0010; 0012

79-11-8
10 suppliers
$27.00/25g

105-39-5
405 suppliers
$10.00/5g
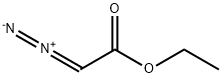
623-73-4
149 suppliers
$27.30/25ML

105-39-5
405 suppliers
$10.00/5g