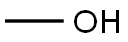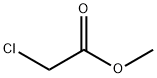
Methyl chloroacetate synthesis
- Product Name:Methyl chloroacetate
- CAS Number:96-34-4
- Molecular formula:C3H5ClO2
- Molecular Weight:108.52
Reaction: Methanol and chloroacetic acid are uniformly mixed in a weight ratio of 0.366:1, heated with stirring, and the esterification reaction is carried out at 105-110 °C. In the reaction process, the ternary azeotrope of methyl chloroacetate, water and methanol is continuously steamed, layered through the ester separator, the separated methanol and water are returned to the reaction pot, and the separated crude ester is made of sodium carbonate. neutralize. The neutralized crude ester is firstly cut out the 130°C fraction by atmospheric distillation, and then subjected to vacuum distillation to collect the 65°C (8kPa) fraction, which is the finished product of methyl chloroacetate. The yield is about 96%.
Yield:96-34-4 98.1%
Reaction Conditions:
with calcium chloride tetrahydrate at 80; for 2 h;Reagent/catalyst;Temperature;
Steps:
2.3. Esterification reaction
General procedure: All esterification experiments were carried out in a 100 mL threeneckedglass flask equipped with a refluxing condenser, a mechanicstirrer and oil bath heating. In a typical experiment, 0.2 mol CA (or otheracid), 20 mL MSH and designed amount of alcohol were charged into theflask. The reaction was timed when the reaction mixture was heated tothe designed temperature (60-80 C). The temperature was controlledwith electrical- heating with accuracy of ± 2 C. The stirring speed wasfixed at 300 rpm which has been verified sufficient to eliminate thediffusion limitation. Upon completing the reaction, the flask was quicklycooled down with water bathing. The products were sampled for analysis.In some cases, the reaction mixture could spontaneously split intotwo phases, an upper organic phase and a bottom aqueous phase. If so,both the organic-phase and the bottom aqueous phase were sampled andanalyzed using gas chromatography and separately. Each experimentwas performed in triplicate and the reported result was the average.
References:
Cui, Hongyou;Li, Zhihe;Wang, Jiangang;Wang, Jinghua;Wang, Ming;Yi, Weiming [Applied Catalysis A: General,2022,vol. 638,art. no. 118640]
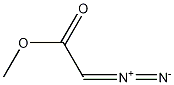
6832-16-2
14 suppliers
inquiry

96-34-4
353 suppliers
$10.00/25g

6832-16-2
14 suppliers
inquiry
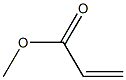
292638-85-8
3 suppliers
inquiry
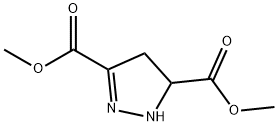
19255-80-2
0 suppliers
inquiry

96-34-4
353 suppliers
$10.00/25g

79-20-9
552 suppliers
$14.00/100g

96-34-4
353 suppliers
$10.00/25g