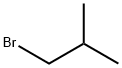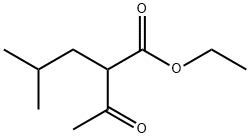
Ethyl 2-isobutylacetoacetate synthesis
- Product Name:Ethyl 2-isobutylacetoacetate
- CAS Number:1522-34-5
- Molecular formula:C10H18O3
- Molecular Weight:186.25
Yield: 56%
Reaction Conditions:
Stage #1:ethyl acetoacetate with sodium hydride in N,N-dimethyl-formamide;mineral oil at 60; for 1 h;
Stage #2:Isobutyl bromide in N,N-dimethyl-formamide;mineral oil at 90; for 3 h;
Steps:
1
Preparation Example 1 - Ethyl 2-acetyl-4-methylpentanoate To a solution of 100 g (0.77 mol) of ethyl acetoacetate in 500 ml of DMF 30.7 g (0.77 mol) of 60% suspension of NaH in mineral oil was added in small portions by vigorous stirring at 60°C. This mixture was additionally stirred for 1 h, and then 105.5 g (0.77 mol) of isobutylbromide was added. The resulting mixture was stirred for 3 h at 90°C, then cooled to room temperature, and 1500 ml of cold water was added. The product was extracted by 3 x 300 ml of dichloromethane. The combined organic extract was evaporated at reduced pressure using rotary evaporator. Fractional rectification of the residue gave the title product, b.p. 75- 80°C/4 mm Hg. Yield 80.5 g (56%).Anal. calc. for Ci0Hi8O3: C, 64.49; H, 9.74. Found: C, 64.20; H, 3.81.1H NMR (CDC13): δ 4.13 (q, J = 7.2 Hz, 2H, CH2Me), 3.44 (dd, J = 8.3 Hz, J = 6.6 Hz, 1H, CHCOMe), 2.16 (s, 3Η, COMe), 1.74 (m, 1Η, CHH'TV), 1.62 (CHH'TV), 1.47 (sept, J = 6.6 Hz, 1H, CHMe2), 1.21 (t, J = 7.2 Hz, 3H, CH2 e), 0.85 (d, J = 6.6 Hz, 6H, CUMe2).
References:
BOREALIS AG;CASTRO, Pascal;RESCONI, Luigi;HUHTANEN, Lauri WO2012/1052, 2012, A2 Location in patent:Page/Page column 36-37

513-38-2
179 suppliers
$60.00/25mL

141-97-9
789 suppliers
$5.00/25g

1522-34-5
71 suppliers
$45.00/25mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1522-34-5
71 suppliers
$45.00/25mg

513-38-2
179 suppliers
$60.00/25mL
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1522-34-5
71 suppliers
$45.00/25mg