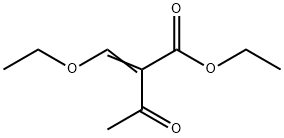
Ethyl 2-(ethoxymethylene)acetoacetate synthesis
- Product Name:Ethyl 2-(ethoxymethylene)acetoacetate
- CAS Number:3788-94-1
- Molecular formula:C9H14O4
- Molecular Weight:186.21

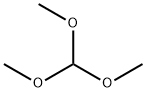
141-97-9
796 suppliers
$5.00/25g

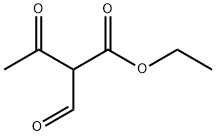
122-51-0
495 suppliers
$10.00/5ml

3788-94-1
175 suppliers
$11.00/1g
Yield:3788-94-1 80%
Reaction Conditions:
with acetic anhydride at 130; for 5 h;
Steps:
2.2.22.a
2.22 Example 22 {prepared via Scheme 12)- 6-[(3-Cyclobutyl-2,3,4,5-tetrahydro-lH-3-benzazepin-7-yl)methyl]-2-(3-methoxyazetidin-l- yl)-6,7-dihydro-5H-pyrrolo[3,4-d]pyrimidin-5-one . iStep a) Intermediate 25Ethyl 2-(ethoxymethylene)-3-oxobutanoate A solution of ethyl 3-oxobutanoate (20 g, 154 mmol), triethylorthoformate (51.1 ml, 307 mmol), and acetic anhydride (43.5 ml, 461 mmol) was heated at 130 °C for 5 h. The reaction was cooled to r.t. and concentrated under reduced pressure to remove triethyl orthoformate and acetic anhydride. The remainder of acetic anhydride and triethyl orthoformate was removed by distillation (30 mbar, 30-70 °C). Ethyl 2-(ethoxymethylene)-3-oxobutanoate was distilled off (6 mbar, 80 °C to 128 °C) to leave ethyl 2-(ethoxymethylene)-3-oxobutanoate as a viscous yellow oil (22.8 g, 80%) NMR (400 MHz, CDC13) δ 7.59 - 7.69 (m, 1H), 4.17 - 4.34 (m, 4H), 2.29 - 2.44 (m, 3H), 1.25 - 1.44 (m, 6H).
References:
WO2011/83316,2011,A1 Location in patent:Page/Page column 52-53
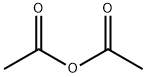
108-24-7
5 suppliers
$14.00/250ML

141-97-9
796 suppliers
$5.00/25g

122-51-0
495 suppliers
$10.00/5ml

3788-94-1
175 suppliers
$11.00/1g
14036-06-7
109 suppliers
$58.00/5g

141-97-9
796 suppliers
$5.00/25g

3788-94-1
175 suppliers
$11.00/1g