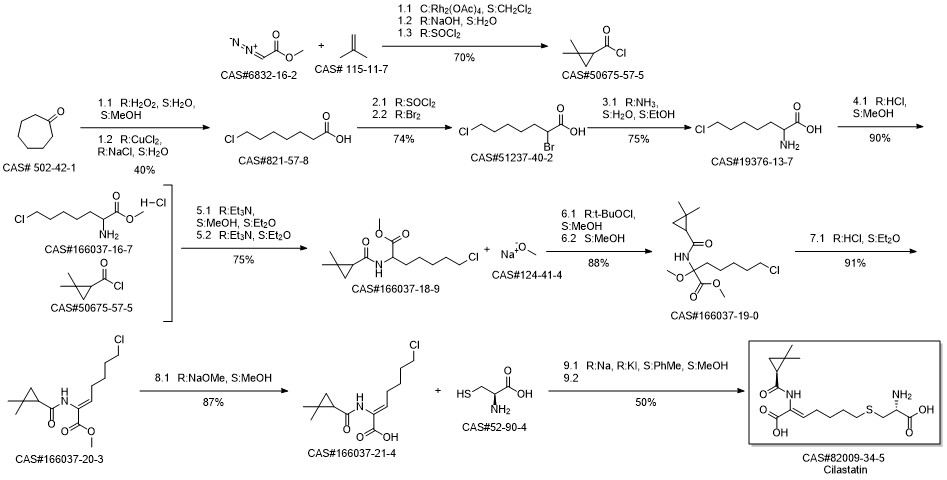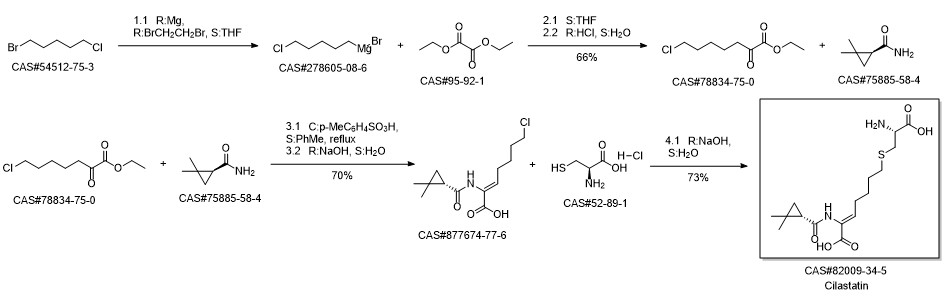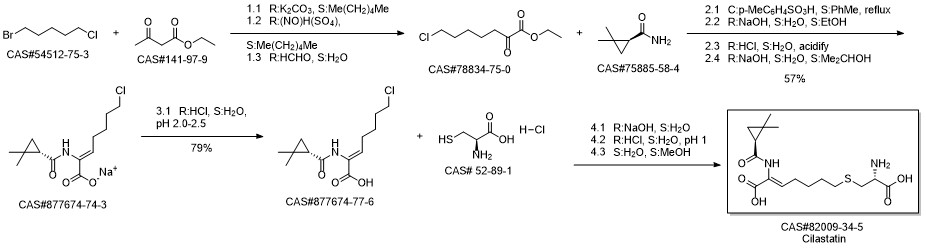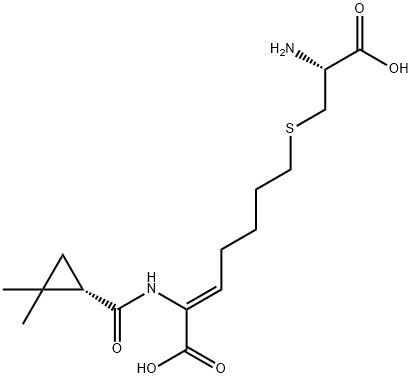
Cilastatin synthesis
- Product Name:Cilastatin
- CAS Number:82009-34-5
- Molecular formula:C16H26N2O5S
- Molecular Weight:358.45
Reference: Graham, Donald W.; Ashton, Wallace T.; Barash, Louis; Brown, Jeannette E.; Brown, Ronald D.; Canning, Laura F.; Chen, Anna; Springer, James P.; Rogers, Edward F. Inhibition of the mammalian β-lactamase renal dipeptidase (dehydropeptidase-I) by Z-2-(acylamino)-3-substituted-propenoic acids. Journal of Medicinal Chemistry. Volume 30. Issue 6. Pages 1074-90. Journal. (1987).
![7-Chloro-2-[[[(1S)-2,2-dimethylcyclopropyl]carbonyl]amino]-2-heptenoic acid](/CAS2/GIF/877674-77-6.gif)
877674-77-6
69 suppliers
inquiry

52-89-1
660 suppliers
$5.00/50mg

82009-34-5
236 suppliers
$39.00/10mg
Yield:82009-34-5 78%
Reaction Conditions:
Stage #1:(Z)-7-chloro-2 ((2s)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid with sodium hydroxide in water at 5; for 0.166667 h;
Stage #2:l-cysteine hydrochloride in water at 45 - 50; for 0.166667 h;Temperature;
Steps:
4.1 (1) Preparation of the compound of formula (VI) cilastatin
At 5° C ,10.5 g of sodium hydroxide was added to 65 g of water,Stirring for 10min,10 g of the compound (Z) -7-chloro-2 ((S) -2,2-dimethylcyclopropylcarboxamido) -2-heptenoic acid represented by the formula (V)Stirring for 10 min;At 45° C ,8 g of cysteine hydrochloride monohydrate was added, stirred for 10 min,The reaction begins at 50 ° C,(Z) -7-chloro-2 ((S) -2,2-dimethylcyclopropylcarboxamido) -2-heptenoic acid in the resulting reaction solution by HPLC was less than 5 % To complete the reaction;After cooling to 20° C ,To the solution was added 223 g of water,Stir,To the solution was slowly added 6M hydrochloric acid solution,Adjust the pH to 2. The obtained solution was added to the Φ45 * 4.0m macroporous adsorption resin column HZ-816, eluted with water, and the conductivity of the collected liquid was less than 100us / cm, and then the aqueous solution was eluted with an aqueous solution of ethanol. The ethanol and water The mass ratio was 15:85 and the elution temperature was 25 ° C. (Z) -7-chloro-2 - ((S) -2,2-dimethylcyclopropylcarboxyl) was added to the above raw material under reduced pressure to collect the material liquid having a conductivity of 100 μ / cm or more 2-heptenoic acid) to obtain the compound of formula (VI). After the addition of 200 g of acetone in the cilastatin, the seeds were incubated at -30 ° C for 6 h, filtered and dried The water content in the obtained cilastatin was less than 3%, and 7.8 g of the pale yellow cilastatin was obtained. The purity was 99.4% by HPLC and the yield was 78%.
References:
Shenzhen Haibin Pharmaceutical Co., Ltd.;Ren, Peng;Zhang, Ming;Ou, Peng;Zhao, Yong;Deng, Huasheng CN106518741, 2017, A Location in patent:Paragraph 0087; 0088; 0092; 0093; 0097; 0098; 0102-0163
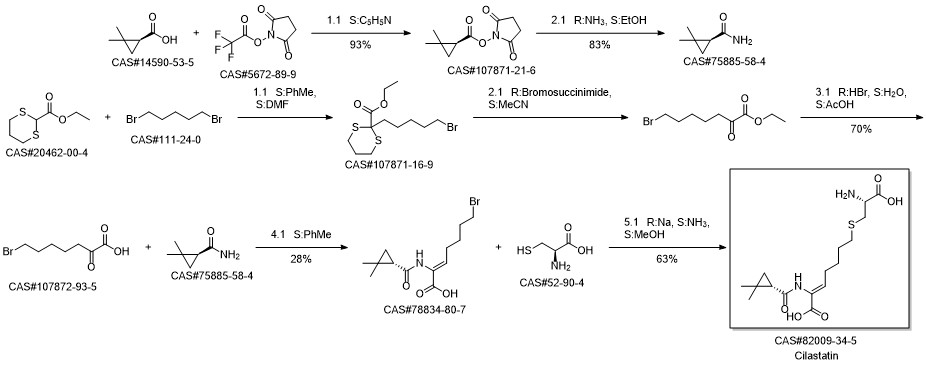
75885-58-4
261 suppliers
$5.00/1g

82009-34-5
236 suppliers
$39.00/10mg
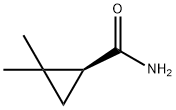
78834-75-0
229 suppliers
$6.00/1g

82009-34-5
236 suppliers
$39.00/10mg
75885-59-5
120 suppliers
$39.00/1g

82009-34-5
236 suppliers
$39.00/10mg
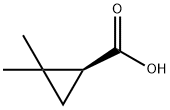
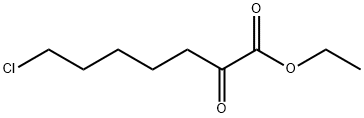
14590-53-5
109 suppliers
$22.00/1g

82009-34-5
236 suppliers
$39.00/10mg