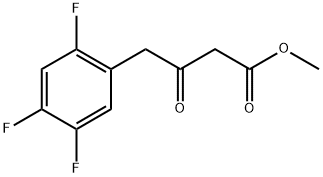
Methyl 3-Oxo-4-(2,4,5-trifluorophenyl)butanoate synthesis
- Product Name:Methyl 3-Oxo-4-(2,4,5-trifluorophenyl)butanoate
- CAS Number:769195-26-8
- Molecular formula:C11H9F3O3
- Molecular Weight:246.19
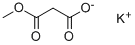
38330-80-2
293 suppliers
$14.00/5g
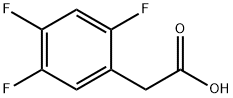
209995-38-0
503 suppliers
$10.00/10 g

769195-26-8
158 suppliers
$12.00/250mg
Yield:769195-26-8 83%
Reaction Conditions:
Stage #1:monomethyl monopotassium malonate with triethylamine in acetonitrile at 30 - 50; for 8.3 h;
Stage #2:(2,4,5-trifluorophenyl)acetic acid with 1,1'-carbonyldiimidazole in acetonitrile at 30; for 3.5 h;
Steps:
1.1
Step-1: Preparation of Methyl 4-(2,4,5-trifluorophenyl)-3-oxobutanoate (Formula III)Monomethylmalonate potassium salt (MMMKS; 122.8 g), triethylamine (264 ml) and acetonitrile (1200 ml) were charged to a 3L round bottom flask (RBF) fitted with condenser, nitrogen inlet, thermometer pocket and overhead stirrer. MgCl2 (65.2 g) was added lot- wise to the above mixture over a period of 15-20 min at 30°C and the mixture was stirred for 10 min. The reaction mixture was heated to 50°C for 8 h at same temperature. After 8 hours, the reaction mixture was cooled to 30°C and marked as Part A. Ι, -carbonyldiimidazole (CDI) (1 10 g) and acetonitrile (250 ml) were charged to a 2L RBF fitted with nitrogen inlet, thermometer pocket, addition funnel and overhead stirrer. To this, a solution of 2,4,5-trifluorophenyl acetic acid (100 g) in acetonitrile (600 ml) was added slowly over a period of a 30 min at 30 °C and the reaction mixture was stirred at 30 °C for 3 h. This solution was marked as part B.The Part B solution was added drop-wise to part-A slurry over a period of 2 hour at 30 °C and the mixture was stirred. The progress of the reaction was monitored by TLC (Mobile phase: n-Hexane: ethyl acetate; 50:50). After 12 h, TLC analysis indicated <5% of un-reacted starting material. The reaction mixture was concentrated under reduced pressure at 50-55°C to get a thick slurry. To this, water (1000 ml) was added and the mixture was cooled to 10-15°C and cone. HC1 (220.6 ml) was added slowly, below 20°C. MTBE (700 ml) was added to the mixture and the mixture was stirred for 30 min. The layers were separated and the aqueous layer was extracted with MTBE (200 ml). The combined organic layers were washed with 7 % aqueous NaHC03 solution (400 ml) followed by washing with brine (200 ml). The organic layer was dried over sodium sulphate and concentrated under vacuum at 50°C to get an oil (120 g). The obtained oil was diluted with isopropyl alcohol (400 ml) and cooled to 20°C. To this water (800 ml) was added slowly over a period of 4 hours at 18-20°C. The slurry was then stirred for 4 hours and filtered. The obtained cake was washed with water (100 ml) and then suck dried to obtain free solid of titled product.Yield: 115 g (83 %); m.p.: 37-39°C; HPLC Purity : > 95 %.H NMR (CDCI3): 3.64 (s, 3H), 3.75 (s, 2H), 3.85 (s, 2H), 6.88-7.11 (m, 2H).Mass spectrum: 247 [M+l] +.
References:
USV LIMITED;SATHE, Dhananjay Govind;DAMLE, Subhash Vishwanath;AROTE, Nitin Dnyaneshwar;AMBRE, Rakesh Ramchandra;SAWANT, Kamlesh Digambar;NAIK, Tushar Anil WO2012/25944, 2012, A2 Location in patent:Page/Page column 29-30
1176895-65-0
6 suppliers
inquiry
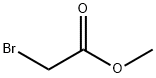
96-32-2
345 suppliers
$15.00/5g

769195-26-8
158 suppliers
$12.00/250mg
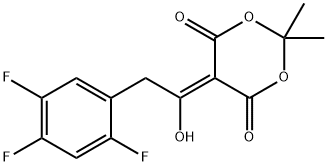
764667-64-3
108 suppliers
$71.00/1g

769195-26-8
158 suppliers
$12.00/250mg
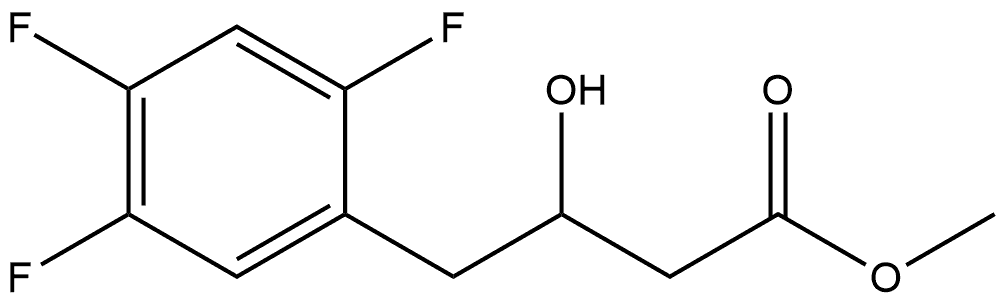
1253056-13-1
0 suppliers
inquiry

769195-26-8
158 suppliers
$12.00/250mg

67-56-1
755 suppliers
$7.29/5ml-f

764667-64-3
108 suppliers
$71.00/1g

769195-26-8
158 suppliers
$12.00/250mg