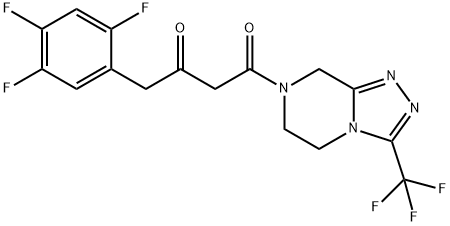
(2Z)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one synthesis
- Product Name:(2Z)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one
- CAS Number:764667-65-4
- Molecular formula:C16H12F6N4O2
- Molecular Weight:406.28
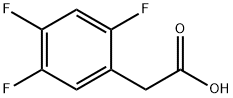
209995-38-0
503 suppliers
$10.00/10 g
![3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride](/CAS/GIF/762240-92-6.gif)
762240-92-6
549 suppliers
$25.00/1 g
![(2Z)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one](/CAS/GIF/764667-65-4.gif)
764667-65-4
252 suppliers
$6.00/1g
Yield:764667-65-4 89%
Reaction Conditions:
Stage #1:(2,4,5-trifluorophenyl)acetic acid with cycl-isopropylidene malonate;N-ethyl-N,N-diisopropylamine;dmap in ISOPROPYLAMIDE at 20 - 40;
Stage #2: with pivaloyl chloride in ISOPROPYLAMIDE at 0 - 5; for 1 - 3 h;
Stage #3:3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride in ISOPROPYLAMIDE at 40 - 70;
Steps:
2; A Scheme 2; Step A : Preparation of 4-oxo-4-[3-(trifluoromethyl)-5,6- dihydro[1,2,4]triazolo[4,3-a]pyrazin- 7 (8H)-YLL-1- (2, 4, 5- TRIFLUOROPHENVL) butan-2-one (2-3)
2,4, 5-TRIFLUOROPHENYLACETIC acid (2-1) (150 g, 0.789 MOL), Meldrum's acid (125 g, 0.868 mol), and 4- (DIMETHYLAMINO) pyridine (DMAP) (7.7 g, 0063 mol) were charged into a 5 L three-neck flask. N, N-DIMETHYLACETAMIDE (DMAC) (525 mL) was added in one portion at room temperature to dissolve the solids. N, N-DIISOPROPYLETHYLAMINE (282 mL, 1.62 mol) was added in one portion at room temperature while maintaining the temperature below 40 °C. Pivaloyl chloride (107 ML, 0.868 mol) was added dropwise over 1 to 2 h while maintaining the temperature between 0 and 5 °C. The reaction mixture was aged at 5 °C for 1 h. Triazole hydrochloride 1-4 (180 g, 0.789 mol) was added in one portion at 40-50 °C. The reaction solution was aged at 70 °C for several h. 5% Aqueous sodium hydrogencarbonate solution (625 ML) was then added dropwise at 20-45 °C. The batch was seeded and aged at 20-30 °C for 1-2 h. Then an additional 525 mL of 5% aqueous sodium hydrogencarbonate solution was added dropwise over 2-3 h. After aging several h at room temperature, the slurry was cooled to 0-5 °C and aged 1 h before filtering the solid. The wet cake was displacement-washed with 20% aqueous DMAC (300 mL), followed by an additional two batches of 20% aqueous DMAC (400 mL), and finally water (400 mL). The cake was suction-dried at room temperature. The isolated yield of final product 2-3 was 89%.
References:
MERCK & CO., INC. WO2005/3135, 2005, A1 Location in patent:Page 12-13

2033-24-1
657 suppliers
$6.00/25g

209995-38-0
503 suppliers
$10.00/10 g
![3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride](/CAS/GIF/762240-92-6.gif)
762240-92-6
549 suppliers
$25.00/1 g
![(2Z)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one](/CAS/GIF/764667-65-4.gif)
764667-65-4
252 suppliers
$6.00/1g
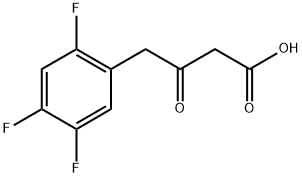
1256815-03-8
26 suppliers
inquiry
![3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine](/CAS/GIF/486460-21-3.gif)
486460-21-3
217 suppliers
$15.00/250mg
![(2Z)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one](/CAS/GIF/764667-65-4.gif)
764667-65-4
252 suppliers
$6.00/1g
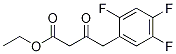
1151240-88-8
39 suppliers
inquiry
![3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine](/CAS/GIF/486460-21-3.gif)
486460-21-3
217 suppliers
$15.00/250mg
![(2Z)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one](/CAS/GIF/764667-65-4.gif)
764667-65-4
252 suppliers
$6.00/1g
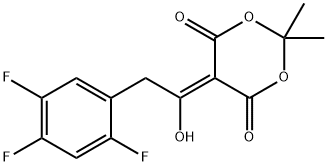
764667-64-3
108 suppliers
$71.00/1g
![3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride](/CAS/GIF/762240-92-6.gif)
762240-92-6
549 suppliers
$25.00/1 g
![(2Z)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one](/CAS/GIF/764667-65-4.gif)
764667-65-4
252 suppliers
$6.00/1g