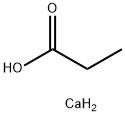
Calcium Propionate synthesis
- Product Name:Calcium Propionate
- CAS Number:4075-81-4
- Molecular formula:C3H8CaO2
- Molecular Weight:116.17
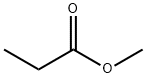
554-12-1
245 suppliers
$21.00/25mL

4075-81-4
677 suppliers
$14.00/25g
Yield:4075-81-4 95.9%
Reaction Conditions:
with water monomer;calcium(II) oxide at 60; for 2.75 h;Temperature;
Steps:
2; 5 Conversion of Methyl Propionate to Calcium Propionate
To a three-necked round bottom flask placed in a heating mantle and equipped with a mechanical stirrer, temperature-measuring thermocouple, and pressure-equalizing dropping funnel, was charged 1600 g of city water followed by 127.7 g (2.28 mole) of lime (95%; Specialty Minerals, Inc) added in 6 min. To this stirred slurry was dropwise added 399 g (4.53 mole) of methyl propionate (99.95%; Lucite) over a period of 0.75 hr. After this addition was complete, the resultant reaction solution was kept at 60° for 2 hr and had a pH of 12.2. The pH was adjusted to 7.2 by addition of 38.8 g of propionic acid. The temperature of the reaction solution was raised to 95° C. to distill volatile components into a receiver. A total of 575.9 g of distillate was collected over a period of 6 hr. Analysis of this distillate gave 426.4 g of water by Karl Fischer titration. The volatile organic constituents of the distillate were analyzed by gas chromatography to give 149.5 g of methanol (103% recovery). The removal of the water by distillation was calculated to ensure that the remaining contents of the round bottom flask was a 26% aqueous solution of calcium propionate. This solution was filtered through diatomaceous earth. Upon cooling, solids were precipitated, removed by filtration, and dried in an oven to give 404.3 g of calcium propionate (95.9% yield) as a white solid. Analysis by standard Ca-EDTA titration gave a purity of 99.8%.
References:
US2022/81384,2022,A1 Location in patent:Paragraph 0042; 0045
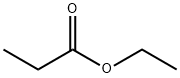
105-37-3
397 suppliers
$10.00/5mL

4075-81-4
677 suppliers
$14.00/25g
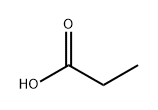
802294-64-0
1 suppliers
inquiry

4075-81-4
677 suppliers
$14.00/25g