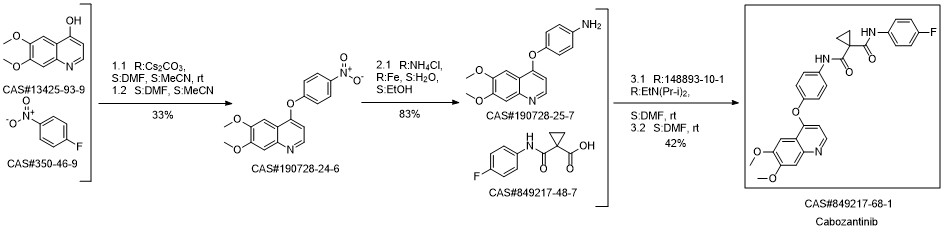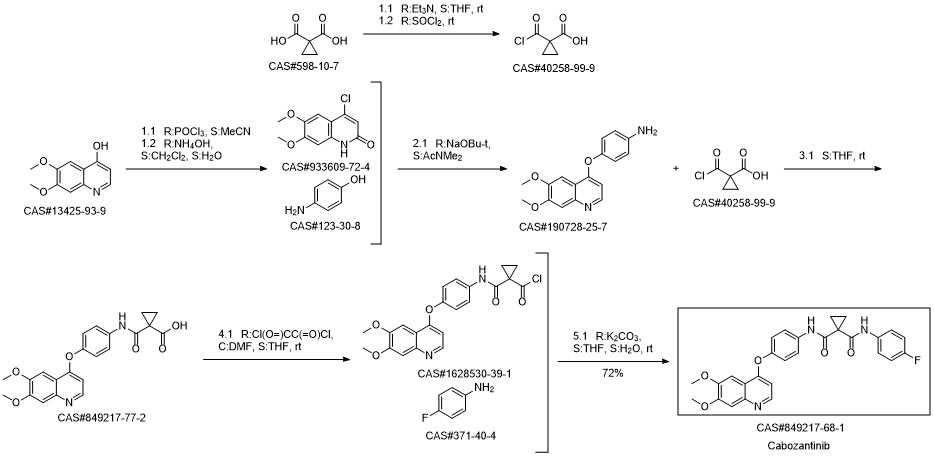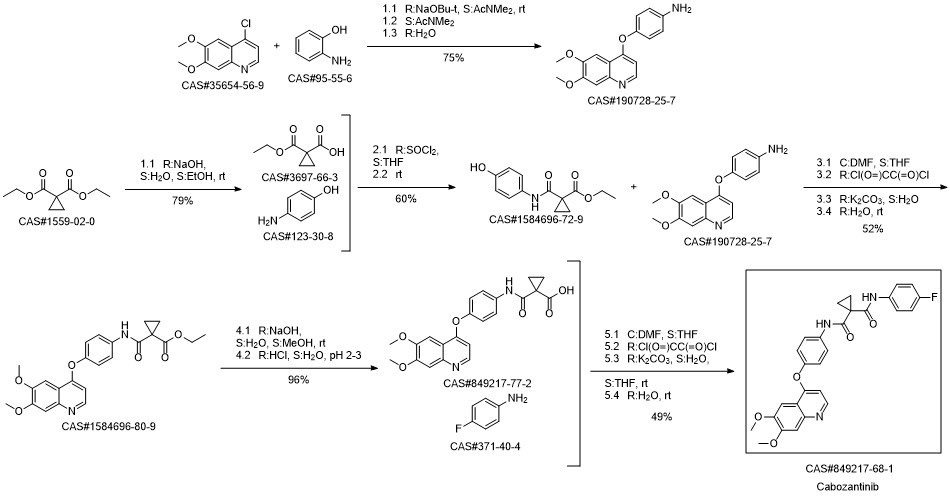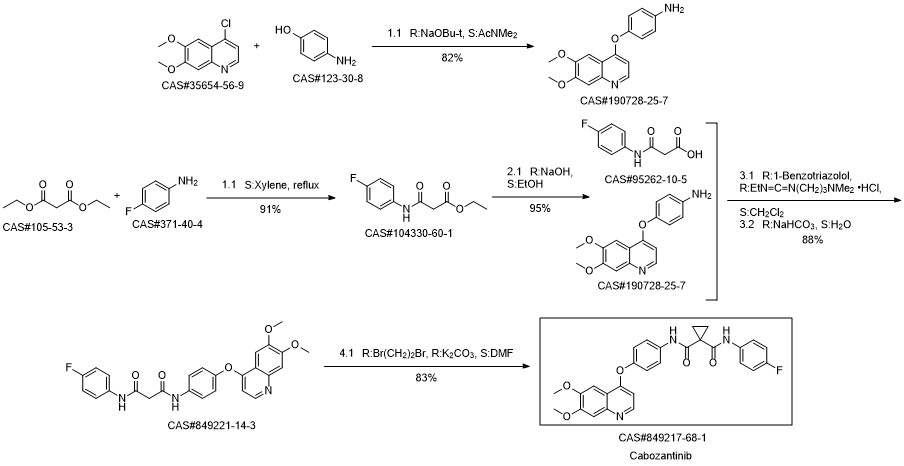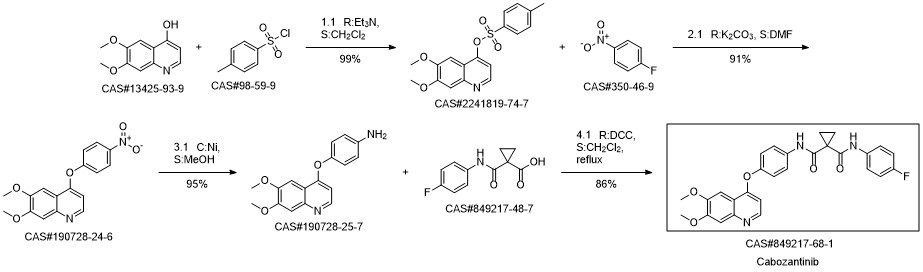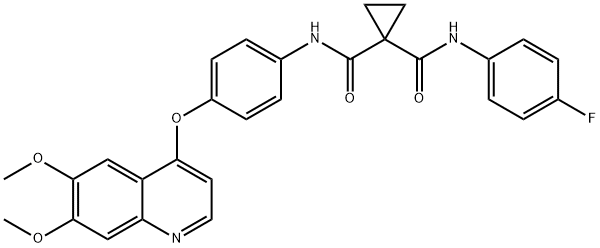
Cabozantinib synthesis
- Product Name:Cabozantinib
- CAS Number:849217-68-1
- Molecular formula:C28H24FN3O5
- Molecular Weight:501.51
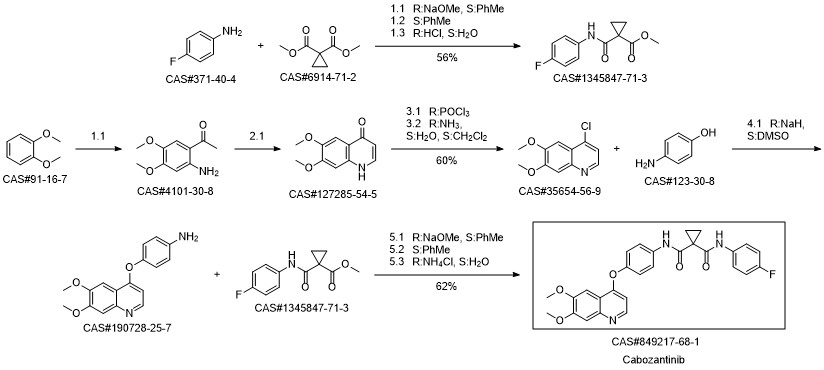
Laus, Gerhard; Schreiner, Erwin; Nerdinger, Sven; Kahlenberg, Volker; Wurst, Klaus; Vergeiner, Stefan; Schottenberger, Herwig. Crystal structures of intermediates in a new synthesis of antitumor drug cabozantinib. Heterocycles. Volume 93. Issue 1, Spec. Issue. Pages 323-332. Journal; Online Computer File. (2016).
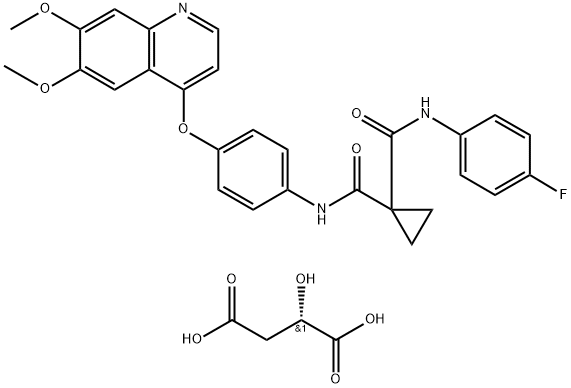
1140909-48-3
325 suppliers
inquiry

849217-68-1
414 suppliers
$7.00/10mg
Yield:849217-68-1 100%
Reaction Conditions:
with sodium hydrogencarbonate in water;ethyl acetate at 45; for 2 h;
Steps:
41 Example 41
A cabozantinib monolauryl sulfate salt was prepared by the following general procedure: a. 37 g of cabozantinib S-malate was added to a co-solvent of (1850 mL/740 mL) of ethyl acetate/l0% aqueous NaHC03 (50V/20V) and stirred at 45°C for 2 hour; (0518) b. The organic layer of the reaction mixture of step (a) was separated and washed twice with 740 mL of purified water (20V x 2); (0519) c. The organic extracts of step (b) were combined and concentrated to obtain cabozantinib free base as a white powder (29.2 g, 100% yield)
References:
WO2019/241504,2019,A1 Location in patent:Page/Page column 110
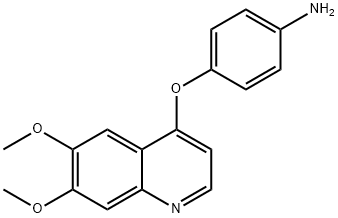
190728-25-7
210 suppliers
$11.00/250mg
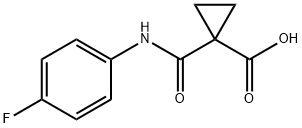
849217-48-7
258 suppliers
$5.00/250mg

849217-68-1
414 suppliers
$7.00/10mg

190728-25-7
210 suppliers
$11.00/250mg
![METHYL 1-[(4-FLUOROPHENYL)CARBAMOYL]CYCLOPROPANECARBOXYLATE](/CAS/20150408/GIF/1345847-71-3.gif)
1345847-71-3
39 suppliers
$40.00/100mg

849217-68-1
414 suppliers
$7.00/10mg
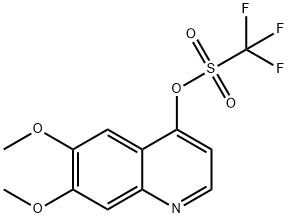
849217-54-5
5 suppliers
inquiry
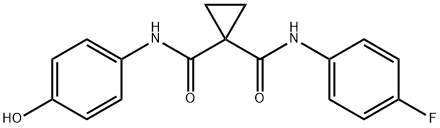
849217-60-3
68 suppliers
$45.00/100mg

849217-68-1
414 suppliers
$7.00/10mg