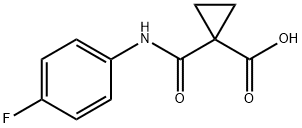
1-((4-Fluorophenyl)carbamoyl)cyclopropanecarboxylic acid synthesis
- Product Name:1-((4-Fluorophenyl)carbamoyl)cyclopropanecarboxylic acid
- CAS Number:849217-48-7
- Molecular formula:C11H10FNO3
- Molecular Weight:223.2
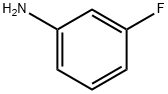
372-19-0
323 suppliers
$10.00/1g
598-10-7
353 suppliers
$7.00/1g

849217-48-7
249 suppliers
$5.00/250mg
Yield:849217-48-7 75.5%
Reaction Conditions:
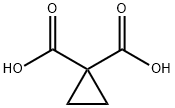
Stage #1:cyclopropane-1,1-dicarboxylic acid with thionyl chloride;triethylamine in dichloromethane for 0.5 h;Cooling with ice;
Stage #2:meta-fluoroaniline in dichloromethane at 20; for 1.5 h;Cooling with ice;
Steps:
1 Example 1. Preparation of 1- (4-fluorophenylcarbamoyl) cyclopropanecarboxylic acid
Compound 2-a (cyclopropane dicarboxylic acid, 0.13 g, 1.00 mmol) was dissolved in dichloromethane (10 ml)After stirring for 10 min in an ice bath, triethylamine (0.42 ml, 3.00 mmol) was added and thionyl chloride (0.143 g, 1.20 mmol) was added slowly to continue stirring atAfter stirring for half an hour under an ice bath, a solution of Compound 1-a (3-fluoroaniline, 0.123 g, 1.0 mmol) in methylene chloride(10ml), continue to stir in the ice bath for half an hour, return to room temperature and stir for 1h (TLC to monitor the reaction is complete). After the reaction is completed,The methylene chloride was recovered under reduced pressure, a saturated aqueous sodium chloride solution (40 ml) was added to the remaining reaction mixture, and the reaction liquid 3 was extracted with ethyl acetateThe combined organic phase was dried over anhydrous sodium sulfate and the solvent was recovered under reduced pressure. 0.21 g of a white solid (Intermediate 1) was obtained.Yield 75.5%;
References:
Hangzhou Xixi Hospital;Liu Shourong;Zhao Yanmei;Kong Limin;Zhou Hongping;Shao Yidan;Cai Zhaobin;Pan Xuwang;Xi Jianjun;Zhang Jiankang;Zhuang Rangxiao;Shi Tingting;Bao Jianfeng;Wang Weiwei;Liu Chuntao;Liu Fei CN106831707, 2017, A Location in patent:Paragraph 0059; 0060; 0061; 0062
![METHYL 1-[(4-FLUOROPHENYL)CARBAMOYL]CYCLOPROPANECARBOXYLATE](/CAS/20150408/GIF/1345847-71-3.gif)
1345847-71-3
38 suppliers
$40.00/100mg

849217-48-7
249 suppliers
$5.00/250mg

598-10-7
353 suppliers
$7.00/1g
371-40-4
409 suppliers
$5.00/5 g

849217-48-7
249 suppliers
$5.00/250mg
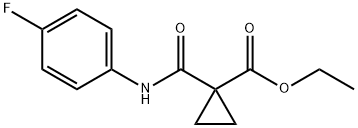
1245931-90-1
5 suppliers
inquiry

849217-48-7
249 suppliers
$5.00/250mg
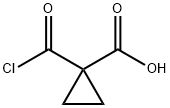
40258-99-9
1 suppliers
inquiry

371-40-4
409 suppliers
$5.00/5 g

849217-48-7
249 suppliers
$5.00/250mg