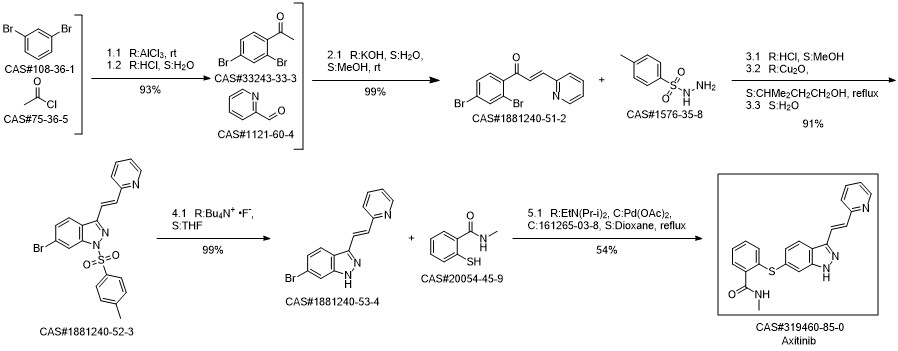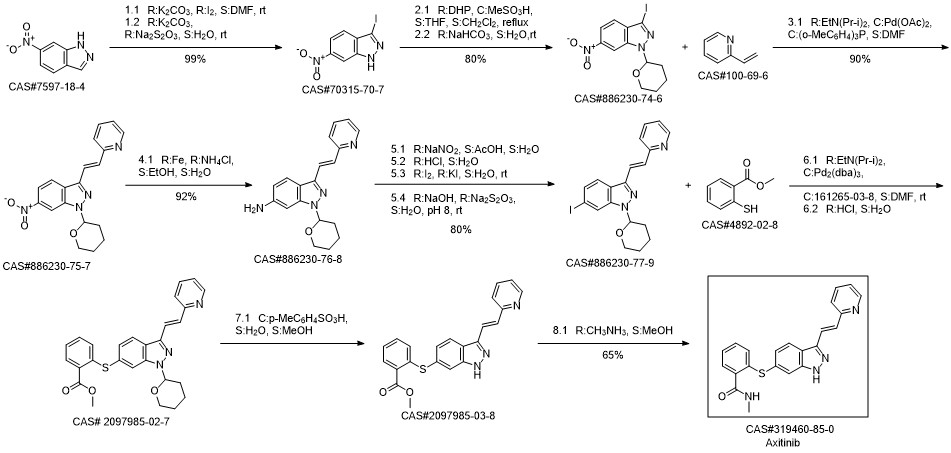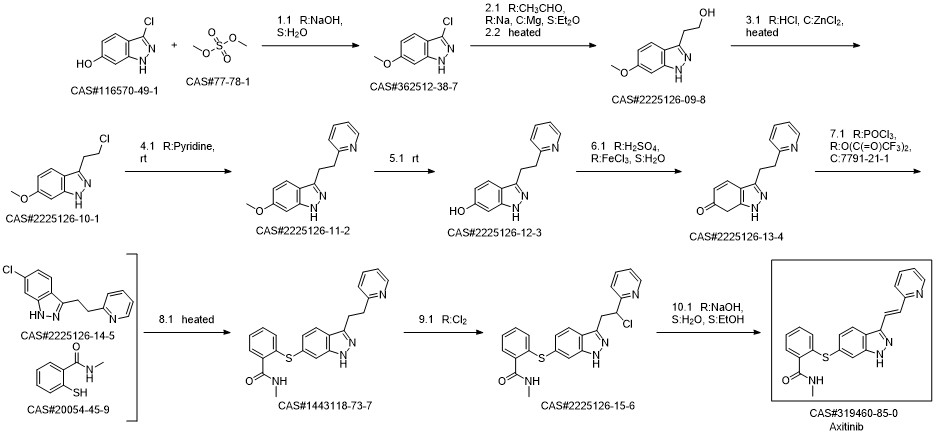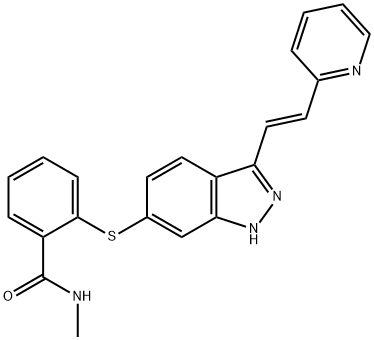
Axitinib synthesis
- Product Name:Axitinib
- CAS Number:319460-85-0
- Molecular formula:C22H18N4OS
- Molecular Weight:386.47
Zhai, Li-Hai; Guo, Li-Hong; Luo, Yang-Hui; Ling, Yang; Sun, Bai-Wang. Effective Laboratory-Scale Preparation of Axitinib by Two CuI-Catalyzed Coupling Reactions. Organic Process Research & Development. Volume 19. Issue 7. Pages 849-857. Journal; Online Computer File. (2015).
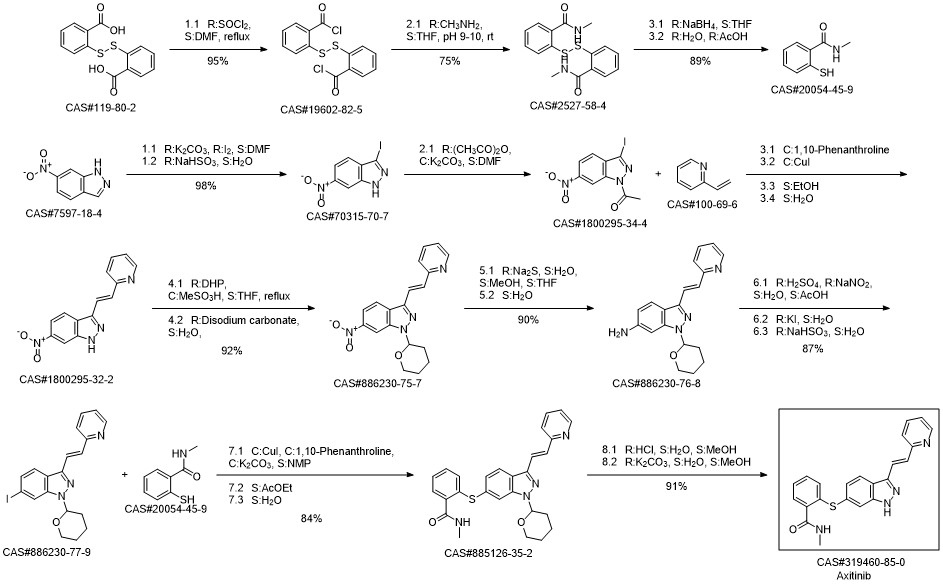
100-69-6
213 suppliers
$14.00/1mL
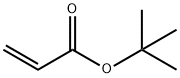
![BenzaMide, 2-[(3-iodo-1H-indazol-6-yl)thio]-N-Methyl-](/CAS/GIF/885126-34-1.gif)
885126-34-1
74 suppliers
$496.32/5MG

319460-85-0
441 suppliers
$39.00/10mg
Yield:319460-85-0 70.68%
Reaction Conditions:
Stage #1: 2-((3-iodo-1H-indazol-6-yl)thio)-N-methylbenzamidewith palladium diacetate;acetic anhydride;N-ethyl-N,N-diisopropylamine;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in 1-methyl-pyrrolidin-2-one at 25 - 50;Inert atmosphere;
Stage #2: 2-vinylpyridine in 1-methyl-pyrrolidin-2-one at 50 - 95; for 12 h;
Steps:
1 Preparation of Axitinib (I)
Take N-methylpyrrolidone (379.11 ml), Palladium (II) acetate (3.64 g, 0.0162 mol) andXantphos (9.32 g, 0.Ol6lmol) into a RB flask at 25-30 °C in nitrogen atmosphere. Charged2-((3-iodo-1H-indazol-6-yl)thio)-N methylbenzamide (HPLC Purity: 99.29%; 165.0 g,0.4031mo1) and diisopropylethylamine(156.62 g, 1.21 mol)under stirring. The reaction was heated to 50°C. Acetic anhydride (83.77 g, 0.8 195 mol) was slowly added and stirred for 2-3 hrs at 50°C. 2-Vinylpyridine (254.97 g, 2.4250 mol) was added slowly, raised the temperature of reaction mass to 90-95°C and maintained for about 12 hrs. Cooled the reaction mixture to 50°C under stirring, diluted with THF (495 ml) and filtered. To the reaction mass1,2-diaminopropane (120.20 g) was added. Stirred at 50°C for 30 mm. Water (1815 ml) was added slowly for 30 mm followed by maintaining temperature 50°C under stirring for 12 hrs. Reaction mass cooled to 15°C and further maintained for 2 hrs, filtered the solid, washed with purified water (495 ml) and THF (163.69 ml). The obtained solid was dried under vacuum to afford crude Axitinib. Yield: ii0 g (70.68%) Chromatographic Purity (By HPLC): 96.3%;XRPD resembles with Fig. II
References:
WO2016/178150,2016,A1 Location in patent:Page/Page column 18

108-86-1
513 suppliers
$10.00/5g
1663-39-4
242 suppliers
$10.00/5g

319460-85-0
441 suppliers
$39.00/10mg
1639137-80-6
38 suppliers
inquiry

319460-85-0
441 suppliers
$39.00/10mg