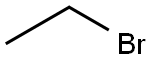
Bromoethane synthesis
- Product Name:Bromoethane
- CAS Number:74-96-4
- Molecular formula:C2H5Br
- Molecular Weight:108.97

64-17-5
724 suppliers
$10.00/50g

74-96-4
441 suppliers
$9.00/5g
Yield:74-96-4 93%
Reaction Conditions:
with 2-pyrrolidonium hydrogen sulfate;hydrogen bromide at 20; for 2 h;Sealed tube;
Steps:
34
Ethanol and hydrobromic acid were reacted to prepare bromoethane. The steps are as follows: In a 500 ml flask, 270 g of 60% hydrobromic acid and 40 g of protonic acid catalyst or ionic liquid were added, maintained the material temperature at about 30°C, while stirring. To the system, 120 g of 95% ethanol was added, the reaction flask was sealed and placed in a water bath to stir the reaction. The temperature continues to rise to boiling state for 1 h. The reaction flask was fed to a rectification plant, bromoethane were separated from the system. The system temperature was raised to 45 ~ 46 degrees C to collect crude ethyl bromide. When the top temperature increases to 60 ° C, collection of ethyl bromide was stopped. The crude ethyl bromide was washed twice with 10% NaOH solution, and re-distilled to obtain the finished product. The results of the reactions of each group are shown in Table 2.
References:
CN104672053,2016,B Location in patent:Paragraph 0056-0060
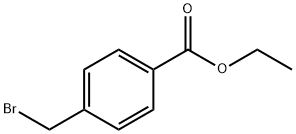
26496-94-6
106 suppliers
$10.00/5g
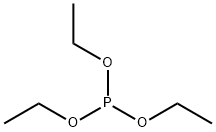
122-52-1
250 suppliers
$9.00/5g

74-96-4
441 suppliers
$9.00/5g
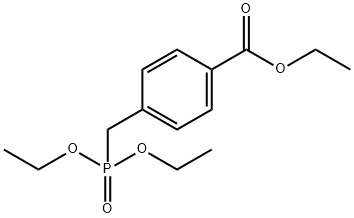
71441-08-2
6 suppliers
inquiry

74-85-1
104 suppliers
$79.00/11l

74-96-4
441 suppliers
$9.00/5g

109-64-8
508 suppliers
$10.00/5g

122-52-1
250 suppliers
$9.00/5g

74-96-4
441 suppliers
$9.00/5g
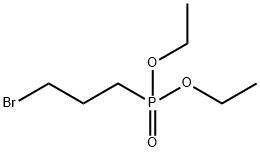
1186-10-3
85 suppliers
$21.00/1g
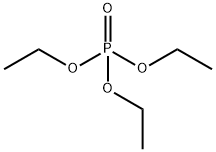
78-40-0
527 suppliers
$5.00/25g

74-96-4
441 suppliers
$9.00/5g