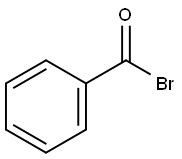
BENZOYL BROMIDE synthesis
- Product Name:BENZOYL BROMIDE
- CAS Number:618-32-6
- Molecular formula:C7H5BrO
- Molecular Weight:185.02

98-88-4
579 suppliers
$10.00/5g

618-32-6
101 suppliers
$39.00/5g
Yield:-
Reaction Conditions:
with lithium bromide at 75; for 4 h;Inert atmosphere;
Steps:
2.2. Synthesis of Cs3Bi2Br9 Double Perovskite
1. Conversion of benzoyl chloride into benzoyl bromide:The reaction was performed in N2 atmosphere. 2 mL ofbenzoyl chloride was added to 1.7369 g of LiBr in 25 mLthree nick flask. The flak was kept on a hot plate at 75 °C for 4 h and the mixture was continuously stirred. The mixture converts from colourless to orange colour, indicating formation of benzoyl bromide. It was cooled down to room temperature (RT) and filtered using a PTFE membranewith 0.45 m pore size [15].
References:
Kumar, Ashish;Rawat, S. S.;Singh, Vidya Nand;Srivastava, Ritu;Swami, Sanjay Kumar [Journal of Nanoscience and Nanotechnology,2020,vol. 20,# 6,p. 3802 - 3808]
65-85-0
1353 suppliers
$10.00/25 g

618-32-6
101 suppliers
$39.00/5g

100-52-7
950 suppliers
$17.30/2g

618-32-6
101 suppliers
$39.00/5g

98-86-2
762 suppliers
$9.00/5g

618-32-6
101 suppliers
$39.00/5g

