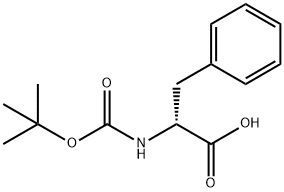
BOC-D-Phenylalanine synthesis
- Product Name:BOC-D-Phenylalanine
- CAS Number:18942-49-9
- Molecular formula:C14H19NO4
- Molecular Weight:265.3
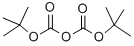
24424-99-5
839 suppliers
$13.50/25G
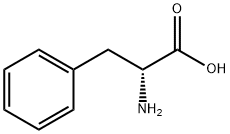
673-06-3
602 suppliers
$14.00/25G

18942-49-9
346 suppliers
$12.50/5G
Yield:18942-49-9 93%
Reaction Conditions:
with guanidine hydrochloride in ethanol at 35 - 40; for 6.5 h;
Steps:
2.4. General procedure for N-tert-butoxycarbonylation of aminoacids and peptides (Table 2, entries 1-12):
General procedure: Amino acid or peptide (1 mmol) was added with stirring to a solution of guanidine hydrochloride (15 mol%) and di-tert-butyl dicarbonate (2.5-3 mmol) in EtOH (1 mL), at 35-40°C. The reaction mixture was continued to stir until a clear solution was obtained. EtOH was evaporated under vacuum and the residue was successively washed with water (2 mL) and hexane or petroleum ether (2 mL) to afford almost pure N-Boc amino acids or N-Boc peptides. If necessary, the crude products could be recrystallized for further purification.
References:
Jahani, Fatemeh;Tajbakhsh, Mahmood;Golchoubian, Hamid;Khaksar, Samad [Tetrahedron Letters,2011,vol. 52,# 12,p. 1260 - 1264] Location in patent:supporting information; experimental part

106454-69-7
236 suppliers
$15.50/1G

18942-49-9
346 suppliers
$12.50/5G

673-06-3
602 suppliers
$14.00/25G
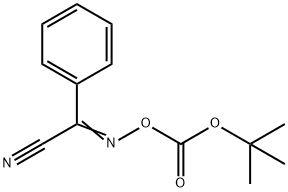
58632-95-4
267 suppliers
$7.00/5g

18942-49-9
346 suppliers
$12.50/5G
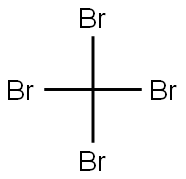
558-13-4
258 suppliers
$10.00/1g
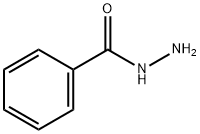
613-94-5
339 suppliers
$5.00/10g

530-62-1
936 suppliers
$6.00/25g

18942-49-9
346 suppliers
$12.50/5G
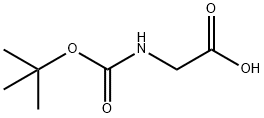
4530-20-5
545 suppliers
$5.00/5 g

18942-49-9
346 suppliers
$12.50/5G