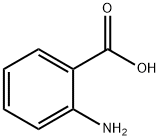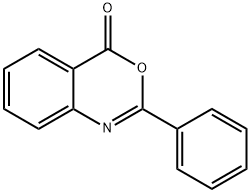
BENTRANIL synthesis
- Product Name:BENTRANIL
- CAS Number:1022-46-4
- Molecular formula:C14H9NO2
- Molecular Weight:223.23
88-67-5
534 suppliers
$5.00/5g

100-47-0
363 suppliers
$14.14/25gm:

1022-46-4
76 suppliers
$90.00/25mg
Yield: 87%
Reaction Conditions:
with 1-butyl-3-methylimidazolium hydroxide in neat (no solvent) for 3 h;Green chemistry;Reagent/catalyst;Solvent;Temperature;
Steps:
4H-benzo[d][1,3-]oxazin-4-one: Procedure
General procedure: A mixture of benzonitrile(2.0 mmol) and o-iodobenzoic acid (2.0 mmol) and [bmIm]OH (35mol) was stirred at room temperature for (3-5) h. After completion of reaction as indicated byTLC, 20mL of water was added to the reaction mixture and stirred well. The product was extracted with ether (3×20 mL). The combined organic layers were dried over anhydrous Na2SO4 to afford the crude product, this crude product was taken to column in order to obtain analytically pure compound 4(a-m)(77-91%). After isolation of the product, the remaining aqueous layer containing the ionic liquid was washed with ether (2x10mL) to remove organic impurity and filtered. The filtrate was extracted with dichloromethane(2×10mL), dried over MgSO4 and evaporated under reduced pressure to afford [bmIm]OH, which was reused five times in subsequent runs without further purification.
References:
Waseem, Malik Abdul;Shireen;Srivastava, Anjali;Srivastava, Arjita;Rahila;Siddiqui [Tetrahedron Letters,2014,vol. 55,# 44,p. 6072 - 6076] Location in patent:supporting information
948-65-2
286 suppliers
$5.00/10g

1022-46-4
76 suppliers
$90.00/25mg
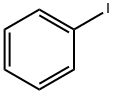
591-50-4
481 suppliers
$10.00/1g
201230-82-2
1 suppliers
inquiry
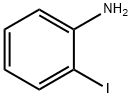
615-43-0
414 suppliers
$5.00/5g

1022-46-4
76 suppliers
$90.00/25mg
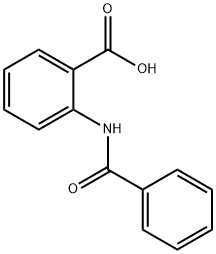
579-93-1
59 suppliers
$22.00/250mg

1022-46-4
76 suppliers
$90.00/25mg