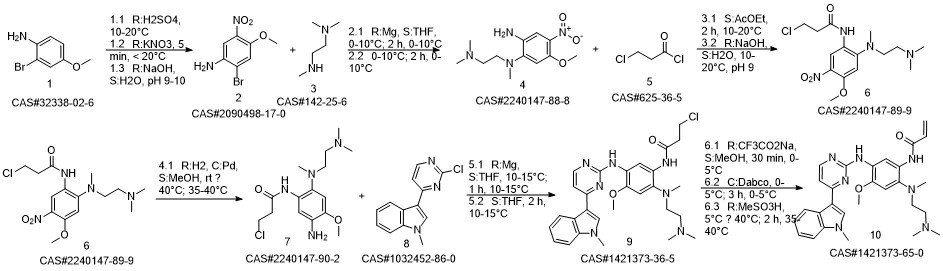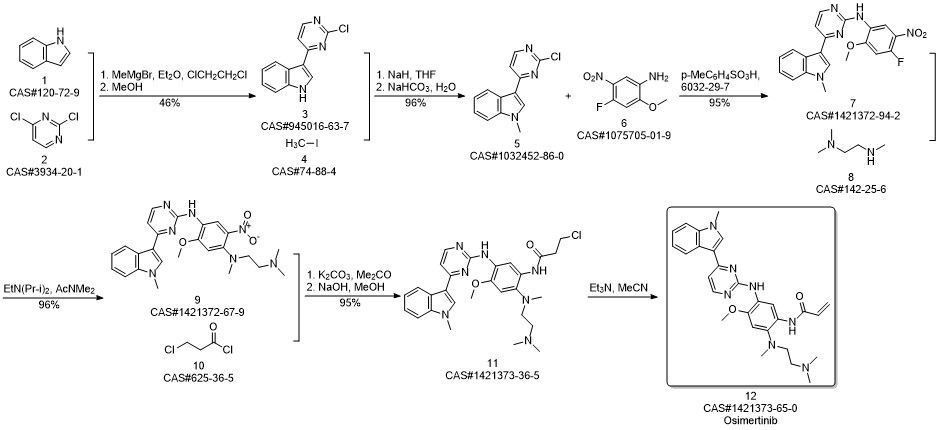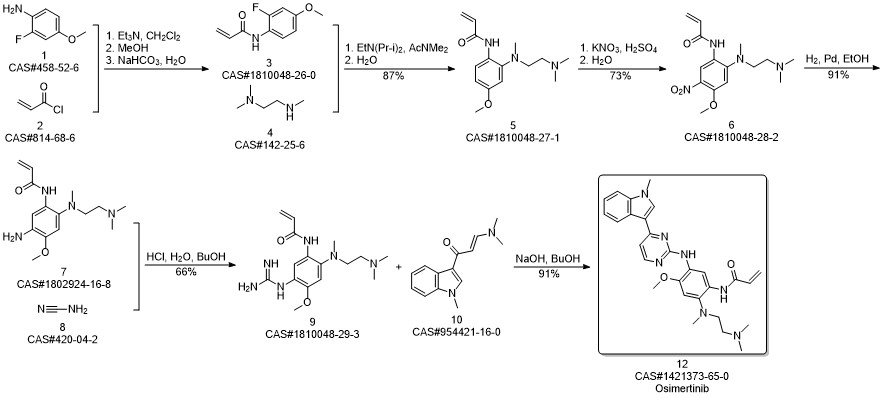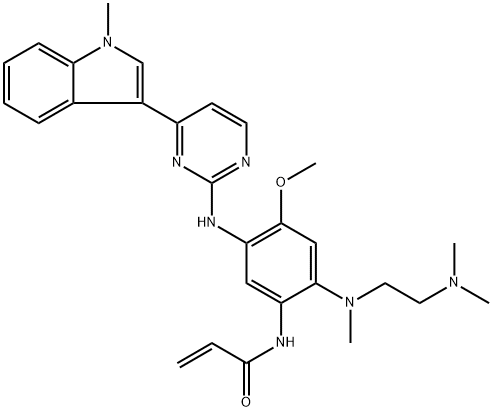
AZD-9291 synthesis
- Product Name:AZD-9291
- CAS Number:1421373-65-0
- Molecular formula:C28H33N7O2
- Molecular Weight:499.61
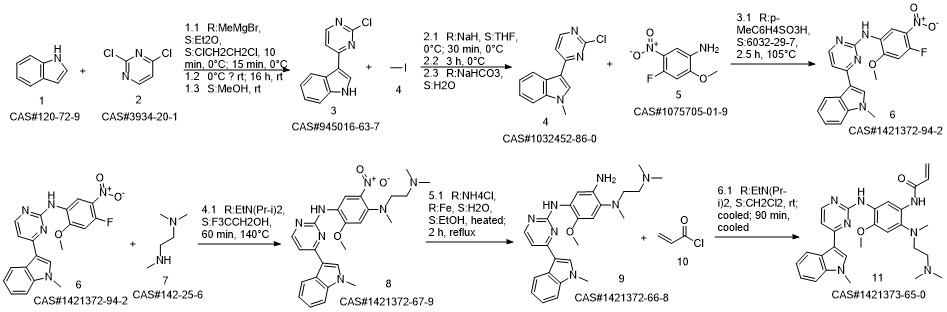
Reference: Finlay MR, Anderton M, Ashton S, Ballard P, Bethel PA, Box MR, Bradbury RH, Brown SJ, Butterworth S, Campbell A, Chorley C, Colclough N, Cross DA, Currie GS, Grist M, Hassall L, Hill GB, James D, James M, Kemmitt P, Klinowska T, Lamont G, Lamont SG, Martin N, McFarland HL, Mellor MJ, Orme JP, Perkins D, Perkins P, Richmond G, Smith P, Ward RA, Waring MJ, Whittaker D, Wells S, Wrigley GL. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem. 2014 Oct 23;57(20):8249-67. doi: 10.1021/jm500973a. Epub 2014 Oct 1. PubMed PMID: 25271963.
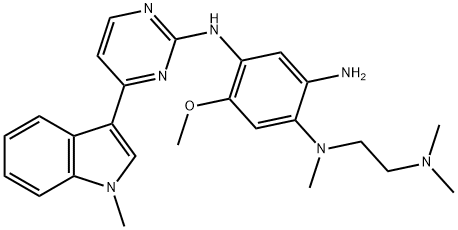
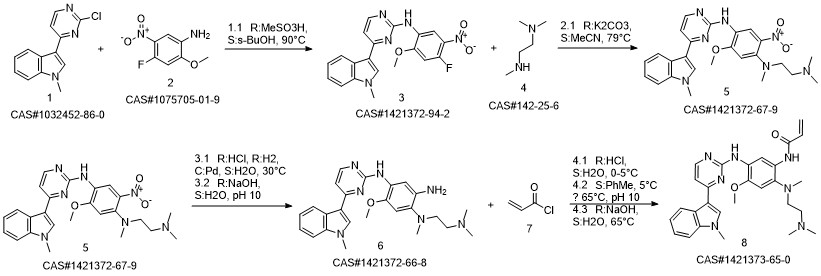
1421372-66-8
208 suppliers
inquiry

79-10-7
701 suppliers
$20.50/500ml

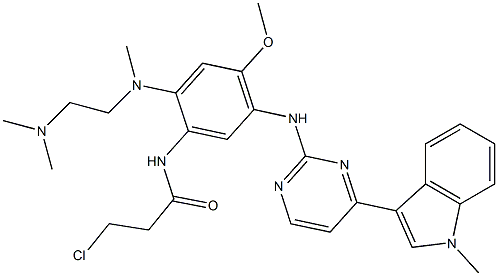
1421373-65-0
370 suppliers
$28.00/1mg
Yield:1421373-65-0 97.9%
Reaction Conditions:
in isopropyl alcohol at 35; for 4 h;Molecular sieve;Microwave irradiation;Temperature;Time;Solvent;
Steps:
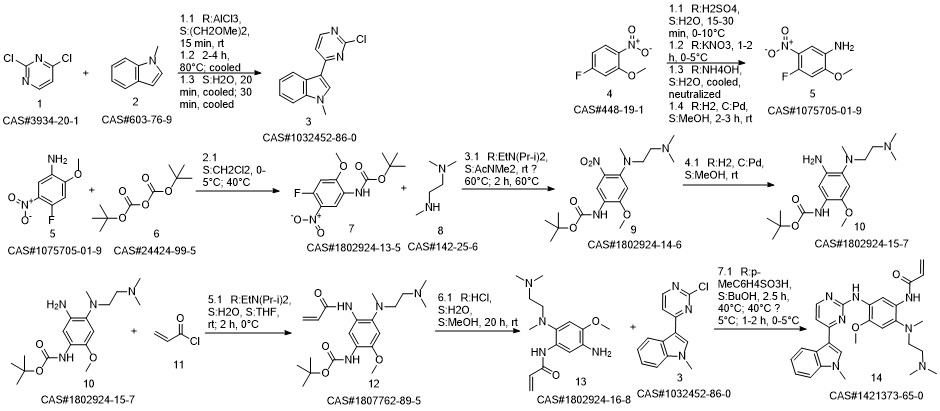
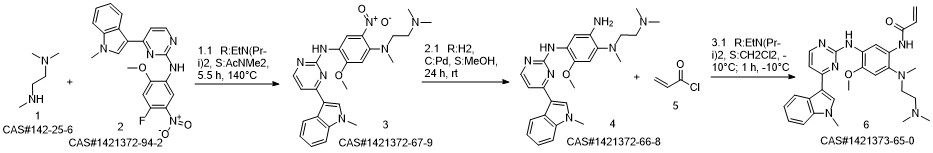
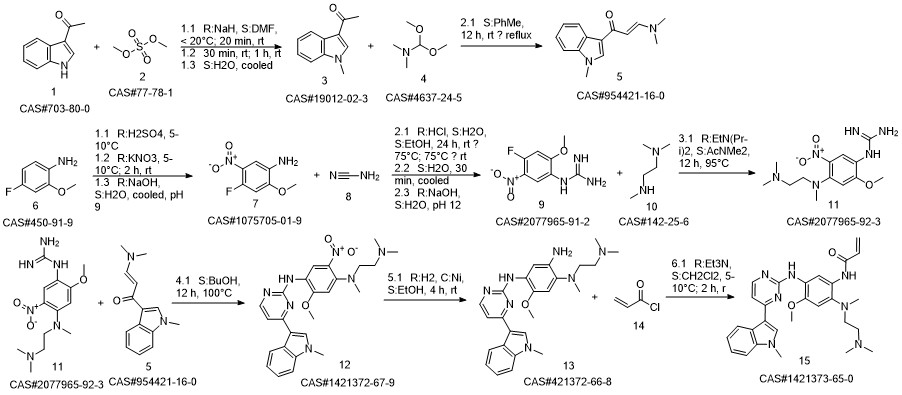
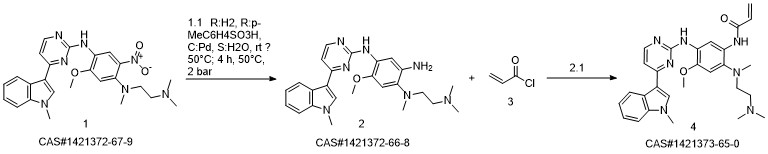
1-8 Example 2
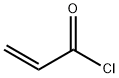
Add to reaction flaskN-2-[[2- (dimethylamino) ethyl] methylamino] -4-methoxy-5-[[4- (1-methyl-1H-indol-3-yl)- 2-pyrimidinyl] amino] aniline (4.45g, 0.010mol),Add acrylic acid (0.86g, 0.012mol),HY molecular sieve (0.66g, 0.003mol),Isopropanol (32mL, 7.2ml / g), microwave heating to 35 ° C,Reaction for 4h, and then dried over anhydrous sodium sulfate,Finally, the solvent was removed by rotary evaporation to obtain 4.88 g of a foamy off-white solid with a yield of 97.9%.
References:
CN110317194,2019,A Location in patent:Paragraph 0027-0042
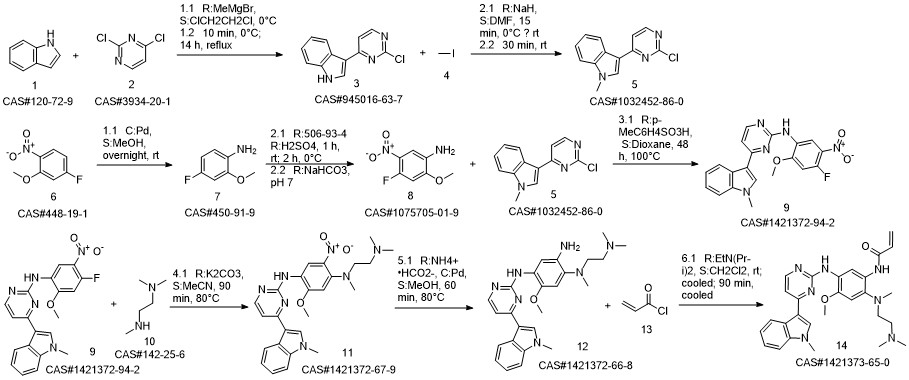
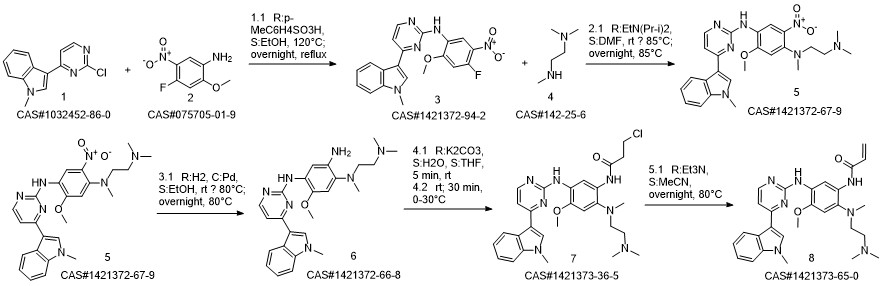
1421373-36-5
22 suppliers
inquiry

1421373-65-0
370 suppliers
$28.00/1mg

1421372-66-8
208 suppliers
inquiry
814-68-6
386 suppliers
$21.21/5gm:

1421373-65-0
370 suppliers
$28.00/1mg

1421372-66-8
208 suppliers
inquiry

625-36-5
370 suppliers
$45.00/5g

1421373-65-0
370 suppliers
$28.00/1mg
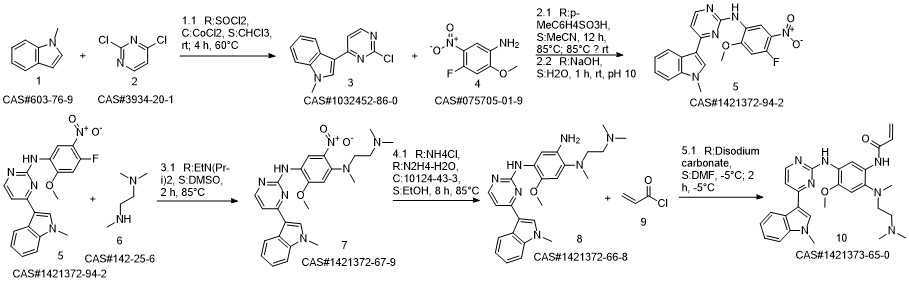
![1,4-BenzenediaMine, N1-[2-(diMethylaMino)ethyl]-5-Methoxy-N1-Methyl-N4-[4-(1-Methyl-1H-indol-3-yl)-2-pyriMidinyl]-2-nitro-](/CAS/20150408/GIF/1421372-67-9.gif)
1421372-67-9
164 suppliers
inquiry

79-10-7
701 suppliers
$20.50/500ml

1421373-65-0
370 suppliers
$28.00/1mg