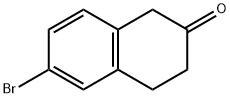
6-Bromo-2-tetralone synthesis
- Product Name:6-Bromo-2-tetralone
- CAS Number:4133-35-1
- Molecular formula:C10H9BrO
- Molecular Weight:225.08

79-37-8
475 suppliers
$17.67/10gm:

1878-68-8
490 suppliers
$6.00/10g

4133-35-1
232 suppliers
$50.00/5g
Yield:4133-35-1 88%
Reaction Conditions:
AlCl3 in N-methyl-acetamide;hexane;dichloromethane
Steps:
1 Step 1
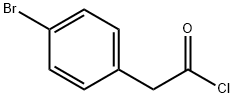
Step 1 Preparation of 6-Bromo-2-tetralone A single neck 3 liter round bottom flask under an Ar atmosphere was charged with 4-bromo-phenyl acetic acid (250.0 g, 1.15 m), methylene chloride (1.5 L), and dimethylformamide (0.5 mL). This magnetically stirred solution was cooled to 0° C. and treated dropwise with oxalyl chloride (156 mL, 1.74 m). The reaction was allowed to reach room temperature and stirred 16 hrs. The reaction was concentrated on a rotary evaporator to approximately 1 L of volume. A separate dry 5 liter 3 neck round bottom flask under Ar, fitted with gas inlet tube, overhead stirrer, and digital thermometer was charged with methylene chloride (1.5 L) and AlCl3 (312.0 g, 2.34 m). This suspension was cooled to 0° C. and stirred while the above solution of acid chloride was added to it slowly via cannula. When the addition was complete, ethylene gas was introduced for 1-2 hrs to the vigorously stirred suspension while maintaining the internal temperature at 15° C. Upon completion by HPLC, the reaction was warmed to room temperature and stirred for 0.5 hrs. The mixture was recooled to 0° C. and cautiously quenched slowly with water (1.5L). The layers were separated, and the aqueous one washed with 500 mL of methylene chloride. The organic portion was washed with 2N aqueous HCl (2*800 mL), brine (400 mL), and saturated aqueous NaHCO3 (2*800 mL). Each aqueous extract was washed with the same 500 mL methylene chloride extract from above. The methylene chloride extracts were combined, dried (Na2 SO4), filtered, and concentrated to approximately 500 mL of volume. This was then added to 5.0L of hexane warmed to 50° C. The methylene chloride was distilled off and the hot solution decanted from an insoluble brown tar. The solution was allowed to cool to 25° C. and placed in the freezer overnight. The precipitate was collected and washed with hexane (200 mL), and dried in vacuo to give 229.0 g of the compound as a pale yellow solid (88%).
References:
Merck & Co., Inc. US5206240, 1993, A
37859-24-8
94 suppliers
$26.10/1g

74-85-1
105 suppliers
$79.00/11l

4133-35-1
232 suppliers
$50.00/5g

1878-68-8
490 suppliers
$6.00/10g

4133-35-1
232 suppliers
$50.00/5g

79-37-8
475 suppliers
$17.67/10gm:

74-85-1
105 suppliers
$79.00/11l

1878-68-8
490 suppliers
$6.00/10g

4133-35-1
232 suppliers
$50.00/5g

74-85-1
105 suppliers
$79.00/11l

1878-68-8
490 suppliers
$6.00/10g

4133-35-1
232 suppliers
$50.00/5g