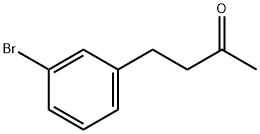
4-(3-bromophenyl)butan-2-one synthesis
- Product Name:4-(3-bromophenyl)butan-2-one
- CAS Number:3506-70-5
- Molecular formula:C10H11BrO
- Molecular Weight:227.1
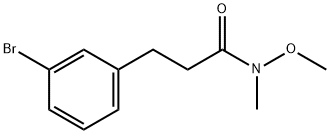
937375-28-5
1 suppliers
inquiry
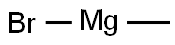
75-16-1
270 suppliers
$12.00/10ml

3506-70-5
22 suppliers
$130.78/1gm:
Yield:3506-70-5 94%
Reaction Conditions:
in tetrahydrofuran at -78 - 0;
Steps:
17
A solution of 3-(3-bromophenyl)-N-methoxy-N-methylpropanamide (Scheme 7, A)(0.500g, 1.84 mmol) and THF (18.0 mL) was cooled to -78° followed by the addition of 3.0 M methylmagnesium bromide (0.623 mL, 1.84 mmol) and allowed to stir at 00C for 2 h. Due to the presence of unreacted starting material, 3.0M methylmagnesium bromide (1.24 mL, 3.68 mmol) was added and allowed to stir overnight at O0C. The reaction was quenched with NH4Cl and the product extracted into DCM, dried ( Na2SO4) and concentrated to give product as a light orange liquid (0.391 g, 94%). 1H NMR (300 MHz, DMSO) δ 7.43 (s, IH)5 7.38 - 7.35 (m5 IH)5 7.25 - 7.21 (m, 2H)5 2.77 (s, 4H)5 2.09 (s, 3H),.m/z (APCI) M (226.9).
References:
WO2007/58601,2007,A1 Location in patent:Page/Page column 80
123-54-6
576 suppliers
$10.00/25ml

932-77-4
137 suppliers
$9.00/1g

3506-70-5
22 suppliers
$130.78/1gm:
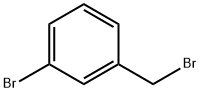
823-78-9
281 suppliers
$8.00/10g

123-54-6
576 suppliers
$10.00/25ml

3506-70-5
22 suppliers
$130.78/1gm:
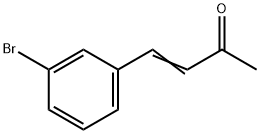
26891-02-1
1 suppliers
inquiry

3506-70-5
22 suppliers
$130.78/1gm:

141-97-9
794 suppliers
$5.00/25g

932-77-4
137 suppliers
$9.00/1g

3506-70-5
22 suppliers
$130.78/1gm: