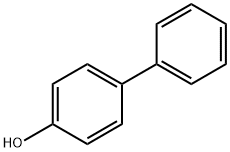
4-Phenylphenol synthesis
- Product Name:4-Phenylphenol
- CAS Number:92-69-3
- Molecular formula:C12H10O
- Molecular Weight:170.21
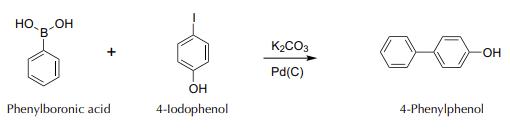
Couple the flask to a water-jacketed condenser, and reflux the mixture on a hot plate with a magnetic stirrer vigorously for 30 min (until a precipitate appears). After this time, switch off the plate and allow to cool to r.t. Add HCl 2 M to an acidic pH (check with indicator paper). Separate the resulting solid, still containing the catalyst, by filtering with a Hirsch funnel. Wash the solid with 10 ml of water. Then, in a Hirsch funnel, add 10 ml of MeOH, and collect the filtrate in a clean container. Add to the resulting MeOH solution 10 ml of deionized water to obtain the precipitate of the product. Purify by recrystallization, heating in a water bath container with the precipitate and the MeOH/H2O mixture. If necessary, add 1 to 2 ml more of hot MeOH, to finish dissolving the solid. Filter under vacuum with a Hirsch funnel, air dry the solid (can recover the next day). Weigh and calculate the yield.
1591-31-7
329 suppliers
$8.00/5g

92-69-3
421 suppliers
$6.00/25g
Yield:92-69-3 99%
Reaction Conditions:
with water;ethylene glycol;potassium hydroxide;copper dichloride in dimethyl sulfoxide at 120; for 24 h;Inert atmosphere;Reagent/catalyst;Temperature;
Steps:
General Procedure A.
To a test tube containing a magnetic bar was added aryl halide (1.0 mmol), CuCl2 (13.4 mg, 0.1 mmol), KOH (336 mg, 6.0 mmol), ethylene glycol(12 μL, 0.2 mmol), and DMSO/H2O (1.0 mL/0.5 mL). After flushing with argon, the mixture was stirred in a preheated oil bath at 120 °C for 24 h. After cooled to ambient temperature, the reaction mixture was distributed in aqueous HCl (5 %) and ethyl acetate. The organic layer was washed with water and brine, dried over anhydrous MgSO4, and concentrated under vacuum. The crude product was further purified by column chromatography (EtOAc/n-Hexane) to provide the phenols.
References:
Kim, Jihye;Battsengel, Oyunsaikhan;Liu, Yajun;Chae, Junghyun [Bulletin of the Korean Chemical Society,2015,vol. 36,# 12,p. 2833 - 2840] Location in patent:supporting information
92-67-1
240 suppliers
$15.90/1g

92-69-3
421 suppliers
$6.00/25g
92-66-0
556 suppliers
$10.00/1g

92-69-3
421 suppliers
$6.00/25g
648930-59-0
3 suppliers
inquiry

92-69-3
421 suppliers
$6.00/25g
4024-24-2
0 suppliers
inquiry

92-69-3
421 suppliers
$6.00/25g