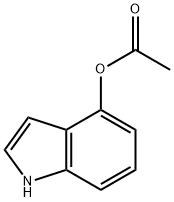
4-Acetoxyindole synthesis
- Product Name:4-Acetoxyindole
- CAS Number:5585-96-6
- Molecular formula:C10H9NO2
- Molecular Weight:175.18
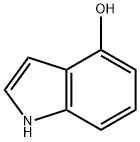
2380-94-1
475 suppliers
$8.00/250mg
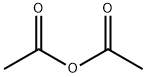
108-24-7
5 suppliers
$14.00/250ML

5585-96-6
223 suppliers
$18.00/1g
Yield:5585-96-6 99.2%
Reaction Conditions:
with pyridine in dichloromethane at 0 - 25;Inert atmosphere;Large scale;
Steps:
2.1
4-hydroxyindole (1eq. limiting reagent) was charged to a vessel under N2 followed by DCM (dichloromethane; 6 vol based on 4-hydroxyindole charge). The reaction was cooled to 0-5°C and pyridine added (1.2 eq) dropwise at 0-5°C. Acetic anhydride (1.1 eq) was added dropwise at 0-5°C and the reaction warmed to 20-25°C for 1 - 1.5 hrs and stirred at 20-25°C for a further 3 hours. The reaction was sampled and analysed for completion. The reaction was then washed three times with 20% aqueous citric acid solution (3 x 3 vol based on 4-hydroxyindole charge) and once with sat. NaHCC-3 (3 vol based on 4-hydroxyindole charge). The DCM solution was dried over MgSC and filtered and the DCM layer concentrated to half volume by distillation. Heptane (6 vol based on 4-hydroxyindole charge) was added and further DC was removed by distillation until full precipitation of the Stage 1 had occurred. The reaction was cooled to 15-25°C and the solids collected by filtration, washing with heptane (1 vol based on 4-hydroxyindole charge) dried under vacuum overnight at 60°C.
References:
WO2019/73379,2019,A1 Location in patent:Paragraph 00237; 00276-00287