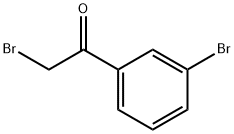
3-BROMOPHENACYL BROMIDE synthesis
- Product Name:3-BROMOPHENACYL BROMIDE
- CAS Number:18523-22-3
- Molecular formula:C8H6Br2O
- Molecular Weight:277.94
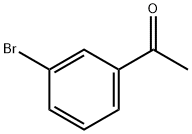
2142-63-4
391 suppliers
$5.00/10g

18523-22-3
184 suppliers
$7.00/1g
Yield:18523-22-3 84%
Reaction Conditions:
with hydrogen bromide;oxygen;2-methylpyridinium nitrate in water at 60; for 10 h;
Steps:
General procedure
General procedure: A mixture of substrate 1 or 3 (5 mmol), HBr (5.5 mmol,40% aqueous solution), and 2-methylpyridinium nitrate (5.5 mmol) was stirred in a reaction flask equipped with a condenser and an O2 balloon at the temperature given inTables 1 and 2. Thin layer chromatography (TLC) and gaschromatography (GC) were used to monitor the progress ofthe reaction. Purification and identification of the productFor 2a-2l. Ten milliliters of saturated Na2CO3 aqueoussolution was added at the end of the reaction, and the resultingmixture was extracted with 20 mL EA three times. Thecombined organic layer was washed with water and driedover Na2SO4; the concentrated residue was purified by columnchromatography (silica gel, petroleum ether (PE): EAfrom 200:1 to 80:1) to afford product 2. For 4a-4e. Ten milliliters of saturated NaHSO3 aqueoussolution was added at the end of the reaction, and the mixturewas extracted with 20 mL EA three times. The combinedorganic layer was then washed with water and driedover Na2SO4; the concentrated residue was purified by columnchromatography (silica gel, PE: dichloromethane(DCM) from 50:1 to 30:1) to afford product 4.
References:
Li, Ming-Fang;Wang, Jian;Ke, Yong-Xin;Pan, Song-Cheng;Yin, Hong;Du, Wenting;Li, Jing-Hua [Journal of Chemical Research,2020,vol. 44,# 5-6,p. 267 - 270]
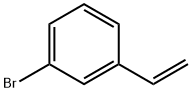
2039-86-3
154 suppliers
$15.00/1g

18523-22-3
184 suppliers
$7.00/1g

2142-63-4
391 suppliers
$5.00/10g

598-55-0
333 suppliers
$6.00/25g

18523-22-3
184 suppliers
$7.00/1g
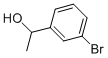
52780-14-0
93 suppliers
$10.00/1g

18523-22-3
184 suppliers
$7.00/1g
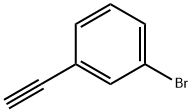
766-81-4
133 suppliers
$12.00/1g

18523-22-3
184 suppliers
$7.00/1g