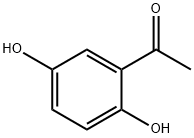
2',5'-Dihydroxyacetophenone synthesis
- Product Name:2',5'-Dihydroxyacetophenone
- CAS Number:490-78-8
- Molecular formula:C8H8O3
- Molecular Weight:152.15
with aluminium chloride(91%) (76%)(63–77%) (55–60%)
with zinc chloride in refluxing acetic acid (quantitative yield)
with boron trifluoride etherate in benzene at reflux (65%).
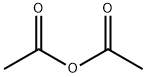
108-24-7
5 suppliers
$14.00/250ML

123-31-9
836 suppliers
$13.00/25g

490-78-8
340 suppliers
$5.00/50mg
Yield:490-78-8 92%
Reaction Conditions:
Stage #1: acetic anhydride;hydroquinone in 1,2-dichloro-ethane at 100; for 4 h;Friedel-Crafts Acylation;
Stage #2: with boron trifluoride diethyl etherate in 1,2-dichloro-ethane at 120; for 3 h;
Steps:
2,5-Dihydroxyacetophenone (7)
1,4-Dihydroxybenzene (5.5 g, 0.05 mol) and dichloroethane (10 mL) were placed in a dry roundbottomed flask, then Ac2O (10 mL, 0.1 mol) was slowly added. The solution was stirred for 4 h at 100 °C until TLC showed that 1,4-dihydroxybenzene had disappeared. BF3-Et2O (9 mL, 0.07 mol) was then added dropwise and the reaction mixture was heated to 120 °C and stirred for another 2-3 h. H2O (40 mL) was then added andthe reaction mixture was extracted with ethyl acetate (50 mL × 2). The combined extracts were washed sequentially with H2O (100 mL × 2), saturated sodium bicarbonate (100 mL × 2) and H2O (100 mL × 1) and then dried with anhydrous sodium sulfate overnight. Removal of the solvent in vacuo to give a solid residue, which was recrystallised from ethanol to give compound 7 as yellow crystals (7.0 g), yield: 92%, m.p.195-198 °C (lit.27 197-199 °C) 1H NMR (500 MHz, DMSO-d6) (δ,ppm): 2.6 (s, 3H, CH3), 6.9 (s, 1H, OH), 7.2-7.3 (m, 3H, ArH), 11.8 (s, 1H, OH).
References:
Cui, Wei;Zhang, Ji;Wang, Qian;Gao, Kai;Zhang, Wei;Yang, Jian [Journal of Chemical Research,2014,vol. 38,# 11,p. 686 - 689]
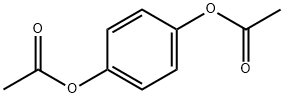
1205-91-0
179 suppliers
$12.00/5g

490-78-8
340 suppliers
$5.00/50mg
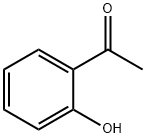
118-93-4
561 suppliers
$10.00/25g

490-78-8
340 suppliers
$5.00/50mg

1201-38-3
325 suppliers
$6.00/5g

490-78-8
340 suppliers
$5.00/50mg
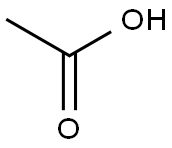
64-19-7
1553 suppliers
$10.00/25ML

123-31-9
836 suppliers
$13.00/25g

490-78-8
340 suppliers
$5.00/50mg