
Hydroquinone synthesis
- Product Name:Hydroquinone
- CAS Number:123-31-9
- Molecular formula:C6H6O2
- Molecular Weight:110.11
Diisopropylbenzene is air oxidized to the intermediate diisopropylbenzene bishydroperoxide. This hydroperoxide is purified by extraction and reacted further to form hydroquinone. The purified product is isolated by filtration and packaged. The process can be almost entirely closed, continuous, computer-controlled, and monitored.
HQcan also be prepared by oxidizing aniline to quinone in the presence of manganese dioxide and sulfuric acid. p-Benzoquinone is then reduced to HQ using iron oxide. The resulting hydroquinone is crystallized and dried. The process occurs in a closed system.
HQis also manufactured by hydroxylation of phenol using hydrogen peroxide as a hydroxylation agent. The reaction is catalyzed by strong mineral acids or ferrous or cobalt salts.
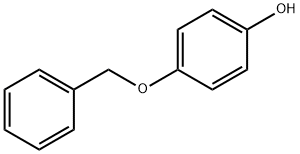
103-16-2
547 suppliers
$5.00/1g

123-31-9
838 suppliers
$13.00/25g
Yield:123-31-9 100%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 50; under 750.075 Torr;Flow reactor;Reagent/catalyst;
Steps:
4.2 Typical procedure for the palladium-catalyzed hydrogenation under the flow conditions (Tables 2 and 3)
General procedure: A solution of the substrates possessing reducible functional groups (1mmol) within the molecule in MeOH (20mL) was flowed at 1mL/min into the catalyst-packed cartridge (10% Pd/C, 10% Pd/HP20, 0.5% Pd/MS3A, or 0.3% Pd/BN; 30mm long-cartridge, ca. 0.3mL volume) under 1, 50, or 80bar hydrogen gas at 25, 50, 75, or 100°C using H-Cube (ThalesNano Nanotechnology Inc.). Catalyst cartridges were filled with 10% Pd/C (99.4mg), 10% Pd/HP20 (101.4mg), 0.5% Pd/MS3A (99.6mg), and 0.3% Pd/BN (99.7mg). The reaction mixture was passed through the catalyst cartridge once and MeOH (the carrier solvent) was concentrated in vacuo to give an analytically pure product. The obtained product was identified by 1H NMR measurement.
References:
Hattori, Tomohiro;Tsubone, Aya;Sawama, Yoshinari;Monguchi, Yasunari;Sajiki, Hironao [Tetrahedron,2014,vol. 70,# 32,p. 4790 - 4798]
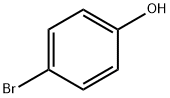
106-41-2
496 suppliers
$13.00/5g

123-31-9
838 suppliers
$13.00/25g
![Benzene, 1,4-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-](/CAS/20210305/GIF/78018-57-2.gif)
78018-57-2
0 suppliers
inquiry

123-31-9
838 suppliers
$13.00/25g
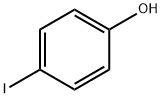
540-38-5
339 suppliers
$10.00/1g

123-31-9
838 suppliers
$13.00/25g

150-78-7
526 suppliers
$5.00/25g

123-31-9
838 suppliers
$13.00/25g