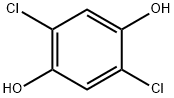
2,5-DICHLOROHYDROQUINONE synthesis
- Product Name:2,5-DICHLOROHYDROQUINONE
- CAS Number:824-69-1
- Molecular formula:C6H4Cl2O2
- Molecular Weight:179
2100-42-7
121 suppliers
$10.00/5g
608-44-6
2 suppliers
inquiry
20103-10-0
38 suppliers
$105.00/50 mg

824-69-1
60 suppliers
$41.79/1g

123-31-9
835 suppliers
$13.00/25g
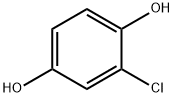
615-67-8
179 suppliers
$13.00/5g
Yield: 0.096 g , 0.048 g , 0.019 g , 1.17 g , 0.105 g
Reaction Conditions:
with aluminum (III) chloride;sodium chloride in melt at 150 - 185; for 0.116667 h;
Steps:
Disproportionation of 2-chloro-1,4-dimethoxyhydroquinone (7) under cycloacylation reaction conditions.
Substrate 7 (1.73 g, 10.0 mmol) was added to the melt of anhydrous AlCl3 (8.54 g,64.0 mmol) and NaCl (1.87 g, 32.0 mmol) with vigorous stirring at 150 °C, the temperature of the mixture was increased to 180-185 °C and maintained for 7 min. Then, the mixture was cooled to room temperature, and diluted with 10% aqueous HCl (60 mL) and distilled water (60 mL). The reaction products were extracted with AcOEt (5×20 mL), the combined extracts were washed with saturated aqueous NaCl (3×15 mL) and dried with Na2SO4, the solvent was evaporated at reduced pressure, the residue was subjected to chromatography on a column with SiO2. The elution with the mixture of hexane-acetone (9 : 1) gave 2,5-dichlorohydroquinone (14) (0.096 g, 29%), colorless crystals, m.p. 168-170 °C, identical to commercial product (Alfa Aesar). 1H NMR (CDCl3), δ: 5.57 (br.s, 2 H, 2 OH); 7.03 (s, 2 H, H(3),H(6)). 13C NMR (CDCl3), δ: 115.0 (C(3), C(6)); 119.9 (C(2),C(5)); 145.7 (C(1), C(4)). 1H NMR (DMSOd6), δ: 6.92 (s, 2 H,H(3), H(6)); 9.75 (s, 2 H, 2 OH). 13C NMR (DMSOd6), δ: 117.0(C(3), C(6)); 118.3 (C(2), C(5)); 145.9 (C(1), C(4)). The elution with the mixture of hexane-acetone (8 : 1) gave2,6dichlorohydroquinone (15) (0.048 g, 14%), colorless crystals,m.p. 163-165 °C (Ref. 42: 164 °C). IR (CDCl3), ν/cm-1: 3601,3549, 2925, 2861, 1499, 1450, 1336, 1304, 1270, 1237, 1194,1175, 1039. 1H NMR (CDCl3), δ: 5.35 (br.s, 1 H, C(1) OH);5.45 (br.s, 1 H, C(4) OH); 6.81 (s, 2 H, H(3), H(5)). 13C NMR(CDCl3), δ: 115.6 (C(3), C(5)); 121.9 (C(2), C(6)); 142.8 (C(1));148.8 (C(4)). 1H NMR (DMSOd6), δ: 6.75 (s, 2 H, H(3), H(5));9.24 (br.s, 1 H, C(1) OH); 9.57 (br.s, 1 H, C(4) OH). 13C NMR(DMSOd6), δ: 115.4 (C(3), C(5)); 123.2 (C(2), C(6)); 141.7(C(1)); 150.8 (C(4)).The elution with the mixture of hexane-acetone (7 : 1) gave2,3dichlorohydroquinone (16) (0.019 g, 6%), colorless crystals,m.p. 142-144 °C (Ref. 43: 144 °C). 1H NMR (DMSOd6), δ:6.76 (s, 2 H, H(5), H(6)); 9.25 (s, 2 H, 2 OH). 13C NMR(DMSOd6), δ: 115.3 (C(5), C(6)); 119.0 (C(2), C(3)); 147.3(C(1), C(4)).The elution with the mixture of hexane-acetone (5 : 1) gave2chlorohydroquinone (12) (1.17 g, 81%), colorless crystals,m.p. 101-102 °C, identical to commercial product (Alfa Aesar).1H NMR (DMSOd6), δ: 6.56 (dd, 1 H, H(5), J = 9.1 Hz,J = 2.8 Hz); 6.71 (d, 1 H, H(3), J = 2.8 Hz); 6.77 (d, 1 H, H(6),J = 9.1 Hz); 9.01 (br.s, 1 H, C(1) OH); 9.21 (br.s, 1 H, C(4)OH). 13C NMR (DMSOd6), δ: 114.8 (C(5)); 116.1 (C(6)); 117.3(C(3)); 119.6 (C(2)); 145.6 (C(1)); 150.3 (C(4)).The elution with the mixture of hexane-acetone (4 : 1) gavehydroquinone (13) (0.105 g, 50%), colorless crystals, m.p.170-172 °C, identical to commercial product (Alfa Aesar).1H NMR (CDCl3), δ: 4.45 (br.s, 2 H, 2 OH); 6.72 (s, 4 H, H(2),H(3), H(5), H(6)). 13C NMR (CDCl3), δ: 116.1 (C(2), C(3),C(5), C(6)); 149.4 (C(1), C(4)). 1H NMR (DMSOd6), δ: 6.56(s, 4 H, H(2), H(3), H(5), H(6)); 8.56 (s, 2 H, 2 OH). 13C NMR(DMSOd6), δ: 115.7 (C(2), C(3), C(5), C(6)); 149.8 (C(1), C(4)).
References:
Novikov;Balaneva;Shestak;Anufriev, V. Ph.;Glazunov [Russian Chemical Bulletin,2016,vol. 65,# 4,p. 993 - 1003][Izv. Akad. Nauk, Ser. Khim.,2016,# 4,p. 993 - 1003,11]
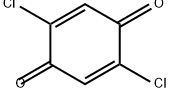
615-93-0
126 suppliers
$32.00/1g

824-69-1
60 suppliers
$41.79/1g
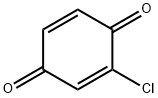
695-99-8
80 suppliers
$40.00/1g

824-69-1
60 suppliers
$41.79/1g
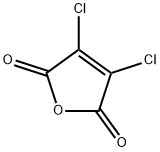
1122-17-4
143 suppliers
$7.00/1g

2100-42-7
121 suppliers
$10.00/5g

608-44-6
2 suppliers
inquiry

20103-10-0
38 suppliers
$105.00/50 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

824-69-1
60 suppliers
$41.79/1g
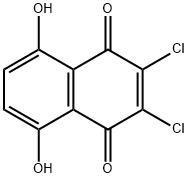
14918-69-5
71 suppliers
$31.00/100mg
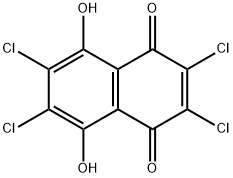
15012-64-3
0 suppliers
inquiry

123-31-9
835 suppliers
$13.00/25g

615-67-8
179 suppliers
$13.00/5g

95-95-4
167 suppliers
$24.00/1g

824-69-1
60 suppliers
$41.79/1g