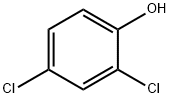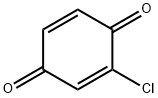
2-CHLORO-1,4-BENZOQUINONE synthesis
- Product Name:2-CHLORO-1,4-BENZOQUINONE
- CAS Number:695-99-8
- Molecular formula:C6H3ClO2
- Molecular Weight:142.54
Yield: 25% , 5%
Reaction Conditions:
with dihydrogen peroxide;Ti-superoxide in water;acetic acid at 60 - 70; for 1.25 h;Product distribution / selectivity;
Steps:
14
Preparation of 2-chloro-1, 4-benzoquinone A mixture of 2, 4-dichlorophenol (5 mmol) and Ti-superoxide catalyst (125 mg, 20% w/w) in acetic acid (5 ml) was heated with stirring at 60-70 C under inert atmosphere. To this reaction mixture was added aq. 30% H202 (20 mmol) drop wise over 15 min. and heated for 1 h. The catalyst was recovered by simple filtration and corresponding quinone formed (25%) was separated by chromatographic purification.Table 1: Ti-superoxide (1) catalyzed oxidation of phenols to quinines and hydroquinones with aq. 30% H202 : a Ex. Substrate t/h Conversion ProductU Selectivity (%) c No. (%) Quinone HQd 1. Phenol 10 22 2 20e 91. 0 2. Phenol 8 66 5 6 92. 4 3. Phenol 7 65 3 60 92.3 4. Phenol 7 68 3.5 63g 92.7 5. Phenol 1 92 88 2.3 95.7 6. o-Cresol 1 99 96 3.0 97.0 7. 7n-Cresol 1 100 99 0.5 99.0 8. 2,6-Dimethylphenol 1 100 97 2.0 97.0 9. 2-'Butylphenol 1 99 97 0. 7 98.0 10. 2, 6-Dibutylphenol 3 70 65 2. 0 93.0 11.4-Chlorophenol 1 57 55-96.5 12.4-Bromophenol 1 61 60-98. 4 13. 4-Iodophenol 1 77 75-97.4 14.2, 4-Dichlorophenol 1 32 25 5. 0 78.1 (a) See examples for detailed experimental procedure; (b) determined by GC analysis of the crude product; (c) selectivity to hydroquinone ; (d) hydroquinone; (e) aq. 10% H202; (f) aq.50% H202; (g) 40% w/w catalyst.
References:
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH WO2005/63664, 2005, A1 Location in patent:Page/Page column 8; Table 1

609-66-5
253 suppliers
$10.00/5g

695-99-8
84 suppliers
$40.00/1g
615-67-8
180 suppliers
$13.00/5g

695-99-8
84 suppliers
$40.00/1g
![Benzene, 2-chloro-1,4-bis[(trimethylsilyl)oxy]-](/CAS/20210305/GIF/67289-08-1.gif)
67289-08-1
0 suppliers
inquiry

695-99-8
84 suppliers
$40.00/1g

108-90-7
658 suppliers
$10.00/25G

695-99-8
84 suppliers
$40.00/1g