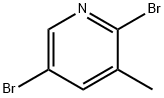
2,5-Dibromo-3-methylpyridine synthesis
- Product Name:2,5-Dibromo-3-methylpyridine
- CAS Number:3430-18-0
- Molecular formula:C6H5Br2N
- Molecular Weight:250.92
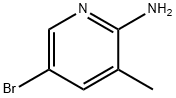
3430-21-5
399 suppliers
$5.00/1g

3430-18-0
365 suppliers
$6.00/5g
Yield:3430-18-0 94%
Reaction Conditions:
with hydrogen bromide;bromine;sodium nitrite in water at -15 - 23; for 3 h;
Steps:
2,5-Dibromo-3-methylpyridine (49)
2,5-Dibromo-3-methylpyridine (49) [0175] (J. Med. Chem. 2007, 50, 3730-3742.) 5-Bromo-3-methylpyridin-2-amine (48, 69 g, 0.37 mol) was suspended in hydrobromic acid (200 mL, 48% in water), and the mixture was cooled to -15° C. Bromine (95 g, 0.59 mol) was added dropwise to the mixture, followed by addition of sodium nitrite (69 g, 1 mol) in water (100 mL). Temperature of the reaction mixture was kept below -15° C. during addition. After addition, the cooling bath was removed and the reaction mixture was stirred for 3 h. The reaction mixture was cooled to -15° C. and quenched with potassium hydroxide (112 g, 2 mol) in water (500 mL). The cooling bath was removed, and the mixture was stirred for 1.5 h. The products were extracted with ethyl acetate (3×300 mL). The combined extracts were washed with water (2×200 mL), saturated aqueous sodium bicarbonate (200 mL), dried with sodium sulfate, and evaporated to dryness. The oily residue was redissolved in chloroform (100 mL), and the solution was filtered through a pad of silica gel, washing with chloroform. The combined filtrates were evaporated to provide 49 as light-yellow solid (87 g, 94%): mp 38-40° C. (lit. (Recl. Tray. Chim. Pays-Bas 1965, 84, 951-964) mp 41-42° C.). 1H NMR (300 MHz, CDCl3) δ 8.22 (d, J=2.4 Hz, 1H), 7.61 (d, J=2.4 Hz, 1H), 2.33 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 148.29, 143.05, 141.15, 137.15, 119.68, 22.06.
References:
CUSHMAN, Mark S.;KISELEV, Evgeny A.;MORRELL, Andrew E. US2014/18360, 2014, A1 Location in patent:Paragraph 0175
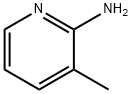
1603-40-3
487 suppliers
$6.00/25g

3430-18-0
365 suppliers
$6.00/5g