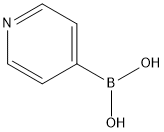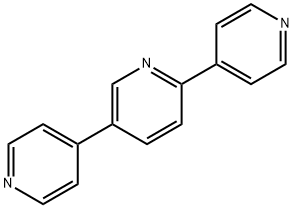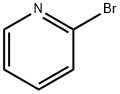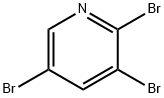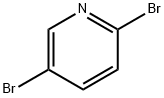
2,5-Dibromopyridine synthesis
- Product Name:2,5-Dibromopyridine
- CAS Number:624-28-2
- Molecular formula:C5H3Br2N
- Molecular Weight:236.89
Add 2-amino-5-bromopyridine (13.0 kg) into a water-cooled solution at 10 °C.
Slowly add 47% aqueous hydrogen bromide (37 L) to the mixture.
Introduce liquid bromine (11 L) into the mixture.
Maintain the reaction temperature below 10 °C.
Prepare a solution of NaNO2 (16.1 kg) and H2O (19 L) and add it dropwise to the mixture while keeping the temperature between 0-5 °C.
Stir the reaction mixture for 30 minutes.
Treat the mixture with a solution of NaOH (28.0 kg) in water (30 L) at a controlled rate below 20-25 °C.
Extract the reaction mixture with diethyl ether (3 × 40 L).
Dry the organic layer with anhydrous Na2SO4.
Remove the desiccant by filtration.
Evaporate the resulting filtrate under reduced pressure to dryness.
Suspend the residue in heptane (10 L).
Collect the target product molecule, 2,5-dibromopyridine, by filtration.
Synthesis method of 2,5-dibromopyridine
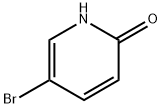
13466-38-1
394 suppliers
$6.00/5g

624-28-2
718 suppliers
$5.00/5g
Yield:624-28-2 88.7%
Reaction Conditions:
with triphenylborane;phosphorus tribromide in 1,2-dichloro-ethane; for 10 h;Inert atmosphere;Reflux;Reagent/catalyst;Solvent;
Steps:
3; 4 Example 4
Under the protection of nitrogen, put 435g and 11.7g (0.048mol) triphenylborane containing 2-hydroxy-5-bromopyridine/1,2-dichloroethane solution in Example 2 into the reaction flask, and add 131g dropwise (0.484mol) Phosphorus tribromide, the temperature is slowly raised to reflux for 10 hours after the dropwise addition is completed. After detecting the completion of the reaction, pour the remaining liquid into 200 mL ice water for quenching, add 10% sodium hydroxide aqueous solution to adjust pH=8.0-9.0, wash the organic phase with water, concentrate dichloromethane under reduced pressure, replace with 55 mL isopropanol, and heat to 50 , add 160mL of water, slowly lower the temperature to precipitate solids, filter, and dry the filter cake to obtain 101.6g of 2,5-dibromopyridine, yield 88.7%, HPLC: 99.5%
References:
CN112679420,2021,A Location in patent:Paragraph 0026-0031
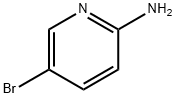
1072-97-5
695 suppliers
$9.00/1g

624-28-2
718 suppliers
$5.00/5g
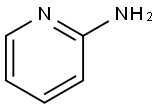
504-29-0
549 suppliers
$5.00/5G

624-28-2
718 suppliers
$5.00/5g